Design and synthesis of water-soluble bioconjugatable trans-AB-porphyrins
- PMID: 20011030
- PMCID: PMC2678728
- DOI: 10.1016/j.tet.2008.08.096
Design and synthesis of water-soluble bioconjugatable trans-AB-porphyrins
Abstract
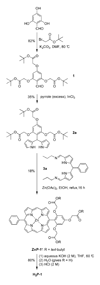
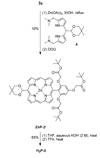
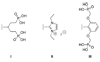
Three free base porphyrins have been prepared that bear a polar and facially encumbering 2,4,6-tris(carboxymethoxy)phenyl motif at one meso (5-) position. The only other substituent (15-position) comprises phenyl, formyl, or p-aminophenyl. The porphyrins exhibit solubility in water (or aqueous buffer solutions) at pH >/=7 and concentrations >1 mM at room temperature. The concise syntheses, water-solubility, and bioconjugatable handle make these porphyrin constructs suitable for biological applications.
Figures







References
-
- Licha K. Top. Curr. Chem. 2002;222:1–29.
-
- Berg K, Selbo PK, Weyergang A, Dietze A, Prasmickaite L, Bonsted A, Engesaeter BØ, Angell-Petersen E, Warloe T, Frandsen N, Høgset A. J. Microscopy. 2005;218:133–147. - PubMed
-
- Kee HL, Nothdurft R, Muthiah C, Diers JR, Fan D, Ptaszek M, Bocian DF, Lindsey JS, Culver JP, Holten D. Photochem. Photobiol. 2008;84 - PubMed
-
- De Rosa SC, Brenchley JM, Roederer M. Nature Med. 2003;9:112–117. - PubMed
-
- Perfetto SP, Chattopadhyay PK, Roederer M. Nature Rev.: Immun. 2004;4:648–655. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
