Glimepiride reduces the expression of PrPc, prevents PrPSc formation and protects against prion mediated neurotoxicity in cell lines
- PMID: 20011040
- PMCID: PMC2784943
- DOI: 10.1371/journal.pone.0008221
Glimepiride reduces the expression of PrPc, prevents PrPSc formation and protects against prion mediated neurotoxicity in cell lines
Abstract
Background: A hallmark of the prion diseases is the conversion of the host-encoded cellular prion protein (PrP(C)) into a disease related, alternatively folded isoform (PrP(Sc)). The accumulation of PrP(Sc) within the brain is associated with synapse loss and ultimately neuronal death. Novel therapeutics are desperately required to treat neurodegenerative diseases including the prion diseases.
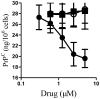
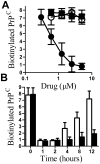
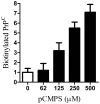
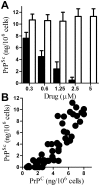
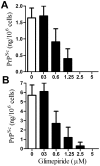
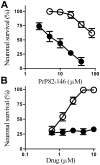
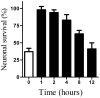
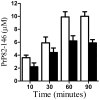
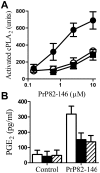
Principal findings: Treatment with glimepiride, a sulphonylurea approved for the treatment of diabetes mellitus, induced the release of PrP(C) from the surface of prion-infected neuronal cells. The cell surface is a site where PrP(C) molecules may be converted to PrP(Sc) and glimepiride treatment reduced PrP(Sc) formation in three prion infected neuronal cell lines (ScN2a, SMB and ScGT1 cells). Glimepiride also protected cortical and hippocampal neurones against the toxic effects of the prion-derived peptide PrP82-146. Glimepiride treatment significantly reduce both the amount of PrP82-146 that bound to neurones and PrP82-146 induced activation of cytoplasmic phospholipase A(2) (cPLA(2)) and the production of prostaglandin E(2) that is associated with neuronal injury in prion diseases. Our results are consistent with reports that glimepiride activates an endogenous glycosylphosphatidylinositol (GPI)-phospholipase C which reduced PrP(C) expression at the surface of neuronal cells. The effects of glimepiride were reproduced by treatment of cells with phosphatidylinositol-phospholipase C (PI-PLC) and were reversed by co-incubation with p-chloromercuriphenylsulphonate, an inhibitor of endogenous GPI-PLC.
Conclusions: Collectively, these results indicate that glimepiride may be a novel treatment to reduce PrP(Sc) formation and neuronal damage in prion diseases.
Conflict of interest statement
Figures


Similar articles
-
Glycosylphosphatidylinositol anchor analogues sequester cholesterol and reduce prion formation.J Biol Chem. 2010 Jul 16;285(29):22017-26. doi: 10.1074/jbc.M110.108548. Epub 2010 Apr 28. J Biol Chem. 2010. PMID: 20427265 Free PMC article.
-
Monoacylated cellular prion protein modifies cell membranes, inhibits cell signaling, and reduces prion formation.J Biol Chem. 2011 Mar 18;286(11):8752-8. doi: 10.1074/jbc.M110.186833. Epub 2011 Jan 6. J Biol Chem. 2011. PMID: 21212283 Free PMC article.
-
Sialic Acid on the Glycosylphosphatidylinositol Anchor Regulates PrP-mediated Cell Signaling and Prion Formation.J Biol Chem. 2016 Jan 1;291(1):160-70. doi: 10.1074/jbc.M115.672394. Epub 2015 Nov 9. J Biol Chem. 2016. PMID: 26553874 Free PMC article.
-
Update on Creutzfeldt-Jakob disease.Curr Opin Neurol. 2004 Dec;17(6):641-7. doi: 10.1097/00019052-200412000-00002. Curr Opin Neurol. 2004. PMID: 15542971 Review.
-
Prion-induced neuronal damage--the mechanisms of neuronal destruction in the subacute spongiform encephalopathies.Curr Top Microbiol Immunol. 2001;253:203-17. doi: 10.1007/978-3-662-10356-2_10. Curr Top Microbiol Immunol. 2001. PMID: 11417136 Review.
Cited by
-
Prion therapeutics: Lessons from the past.Prion. 2022 Dec;16(1):265-294. doi: 10.1080/19336896.2022.2153551. Prion. 2022. PMID: 36515657 Free PMC article. Review.
-
Reduced cell proliferation and neuroblast differentiation in the dentate gyrus of high fat diet-fed mice are ameliorated by metformin and glimepiride treatment.Neurochem Res. 2011 Dec;36(12):2401-8. doi: 10.1007/s11064-011-0566-3. Epub 2011 Aug 5. Neurochem Res. 2011. PMID: 21818657
-
Glimepiride reduces CD14 expression and cytokine secretion from macrophages.J Neuroinflammation. 2014 Jun 21;11:115. doi: 10.1186/1742-2094-11-115. J Neuroinflammation. 2014. PMID: 24952384 Free PMC article.
-
Exploring immunotherapeutic strategies for neurodegenerative diseases: a focus on Huntington's disease and Prion diseases.Acta Pharmacol Sin. 2025 Jun;46(6):1511-1538. doi: 10.1038/s41401-024-01455-w. Epub 2025 Jan 31. Acta Pharmacol Sin. 2025. PMID: 39890942 Review.
-
Anchorless risk or released benefit? An updated view on the ADAM10-mediated shedding of the prion protein.Cell Tissue Res. 2023 Apr;392(1):215-234. doi: 10.1007/s00441-022-03582-4. Epub 2022 Jan 27. Cell Tissue Res. 2023. PMID: 35084572 Free PMC article. Review.
References
-
- Prusiner SB, McKinley MP, Bowman KA, Bolton DC, Bendheim PE, et al. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983;35:349–358. - PubMed
-
- Jeffrey M, Halliday WG, Bell J, Johnston AR, MacLeod NK, et al. Synapse loss associated with abnormal PrP precedes neuronal degeneration in the scrapie-infected murine hippocampus. NeuropatholApplNeurobiol. 2000;26:41–54. - PubMed
-
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

