Extramitochondrial Ca2+ in the nanomolar range regulates glutamate-dependent oxidative phosphorylation on demand
- PMID: 20011041
- PMCID: PMC2784944
- DOI: 10.1371/journal.pone.0008181
Extramitochondrial Ca2+ in the nanomolar range regulates glutamate-dependent oxidative phosphorylation on demand
Abstract
We present unexpected and novel results revealing that glutamate-dependent oxidative phosphorylation (OXPHOS) of brain mitochondria is exclusively and efficiently activated by extramitochondrial Ca(2+) in physiological concentration ranges (S(0.5) = 360 nM Ca(2+)). This regulation was not affected by RR, an inhibitor of the mitochondrial Ca(2+) uniporter. Active respiration is regulated by glutamate supply to mitochondria via aralar, a mitochondrial glutamate/aspartate carrier with regulatory Ca(2+)-binding sites in the mitochondrial intermembrane space providing full access to cytosolic Ca(2+). At micromolar concentrations, Ca(2+) can also enter the intramitochondrial matrix and activate specific dehydrogenases. However, the latter mechanism is less efficient than extramitochondrial Ca(2+) regulation of respiration/OXPHOS via aralar. These results imply a new mode of glutamate-dependent OXPHOS regulation as a demand-driven regulation of mitochondrial function. This regulation involves the mitochondrial glutamate/aspartate carrier aralar which controls mitochondrial substrate supply according to the level of extramitochondrial Ca(2+).
Conflict of interest statement
Figures
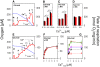
 ) as (○), but mitochondria were uncoupled by 50 nM FCCP from the beginning of experiments, and then Ca2+ titration was performed. (▴) as (○), but Ca2+ was adjusted at the beginning of experiments as indicated. Thereafter, 100 µM ADP was added, causing short transitions between the active and resting states of respiration. After reaching state 4 respiration, FCCP titrations were performed to uncouple respiration and ATP generation. Maximum respiration rates were obtained at 60 or 80 nM FCCP and were plotted against the Ca2+
free value for the respective incubation. Data are means±S.E. of 4 independent experiments.
) as (○), but mitochondria were uncoupled by 50 nM FCCP from the beginning of experiments, and then Ca2+ titration was performed. (▴) as (○), but Ca2+ was adjusted at the beginning of experiments as indicated. Thereafter, 100 µM ADP was added, causing short transitions between the active and resting states of respiration. After reaching state 4 respiration, FCCP titrations were performed to uncouple respiration and ATP generation. Maximum respiration rates were obtained at 60 or 80 nM FCCP and were plotted against the Ca2+
free value for the respective incubation. Data are means±S.E. of 4 independent experiments.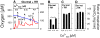

Similar articles
-
Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs).Biochim Biophys Acta. 2016 Aug;1857(8):1158-1166. doi: 10.1016/j.bbabio.2016.04.003. Epub 2016 Apr 7. Biochim Biophys Acta. 2016. PMID: 27060251 Review.
-
The regulation of OXPHOS by extramitochondrial calcium.Biochim Biophys Acta. 2010 Jun-Jul;1797(6-7):1018-27. doi: 10.1016/j.bbabio.2010.02.005. Epub 2010 Feb 6. Biochim Biophys Acta. 2010. PMID: 20144582 Review.
-
Cytosolic, but not matrix, calcium is essential for adjustment of mitochondrial pyruvate supply.J Biol Chem. 2020 Apr 3;295(14):4383-4397. doi: 10.1074/jbc.RA119.011902. Epub 2020 Feb 24. J Biol Chem. 2020. PMID: 32094224 Free PMC article.
-
Impaired regulation of brain mitochondria by extramitochondrial Ca2+ in transgenic Huntington disease rats.J Biol Chem. 2008 Nov 7;283(45):30715-24. doi: 10.1074/jbc.M709555200. Epub 2008 Jul 7. J Biol Chem. 2008. PMID: 18606820 Free PMC article.
-
A Ca2+-Dependent Mechanism Boosting Glycolysis and OXPHOS by Activating Aralar-Malate-Aspartate Shuttle, upon Neuronal Stimulation.J Neurosci. 2022 May 11;42(19):3879-3895. doi: 10.1523/JNEUROSCI.1463-21.2022. Epub 2022 Apr 6. J Neurosci. 2022. PMID: 35387872 Free PMC article.
Cited by
-
Fluctuations in Cytosolic Calcium Regulate the Neuronal Malate-Aspartate NADH Shuttle: Implications for Neuronal Energy Metabolism.Neurochem Res. 2015 Dec;40(12):2425-30. doi: 10.1007/s11064-015-1652-8. Epub 2015 Jul 3. Neurochem Res. 2015. PMID: 26138554 Review.
-
Structure and Function of the Mammalian Neuromuscular Junction.Compr Physiol. 2022 Aug 11;12(4):3731-3766. doi: 10.1002/cphy.c210022. Compr Physiol. 2022. PMID: 35950651 Free PMC article.
-
Role of DJ-1 in the mechanism of pathogenesis of Parkinson's disease.J Bioenerg Biomembr. 2019 Jun;51(3):175-188. doi: 10.1007/s10863-019-09798-4. Epub 2019 May 3. J Bioenerg Biomembr. 2019. PMID: 31054074 Free PMC article. Review.
-
Phototransduction Influences Metabolic Flux and Nucleotide Metabolism in Mouse Retina.J Biol Chem. 2016 Feb 26;291(9):4698-710. doi: 10.1074/jbc.M115.698985. Epub 2015 Dec 16. J Biol Chem. 2016. PMID: 26677218 Free PMC article.
-
Substrate- and Calcium-Dependent Differential Regulation of Mitochondrial Oxidative Phosphorylation and Energy Production in the Heart and Kidney.Cells. 2021 Dec 31;11(1):131. doi: 10.3390/cells11010131. Cells. 2021. PMID: 35011693 Free PMC article.
References
-
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955;217(1):409–427. - PubMed
-
- Gellerich FN, Schlame M, Bohnensack R, Kunz W. Dynamic compartmentation of adenine nucleotides in the mitochondrial intermembrane space of rat heart mitochondria. Biochim Biophys Acta. 1987;890(2):117–126. - PubMed
-
- Seppet EK, Kaambre T, Sikk P, Tiivel T, Vija H, et al. Functional complexes of mitochondria with Ca,MgATPases of myofibrils and sarcoplasmic reticulum in muscle cells. Biochim Biophys Acta. 2001;1504(2–3):379–395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

