The immunogenic SigA enterotoxin of Shigella flexneri 2a binds to HEp-2 cells and induces fodrin redistribution in intoxicated epithelial cells
- PMID: 20011051
- PMCID: PMC2785471
- DOI: 10.1371/journal.pone.0008223
The immunogenic SigA enterotoxin of Shigella flexneri 2a binds to HEp-2 cells and induces fodrin redistribution in intoxicated epithelial cells
Abstract
Background: We have previously shown that the enterotoxin SigA which resides on the she pathogenicity island (PAI) of S. flexneri 2a is an autonomously secreted serine protease capable of degrading casein. We have also demonstrated that SigA is cytopathic for HEp-2 cells and plays a role in the intestinal fluid accumulation associated with S. flexneri infections.
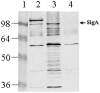
Methods/principal findings: In this work we show that SigA binds specifically to HEp-2 cells and degrades recombinant human alphaII spectrin (alpha-fodrin) in vitro, suggesting that the cytotoxic and enterotoxic effects mediated by SigA are likely associated with the degradation of epithelial fodrin. Consistent with our data, this study also demonstrates that SigA cleaves intracellular fodrin in situ, causing its redistribution within cells. These results strongly implicate SigA in altering the cytoskeleton during the pathogenesis of shigellosis. On the basis of these findings, cleavage of fodrin is a novel mechanism of cellular intoxication for a Shigella toxin. Furthermore, information regarding immunogenicity to SigA in infected patients is lacking. We studied the immune response of SigA from day 28 post-challenge serum of one volunteer from S. flexneri 2a challenge studies. Our results demonstrate that SigA is immunogenic following infection with S. flexneri 2a.
Conclusions: This work shows that SigA binds to epithelial HEp-2 cells as well as being able to induce fodrin degradation in vitro and in situ, further extending its documented role in the pathogenesis of Shigella infections.
Conflict of interest statement
Figures


References
-
- Sasakawa, C Molecular basis of pathogenicity of Shigella. Rev Med Microbiol. 1995;6:257–266.
-
- Henderson IR, Navarro-Garcia F, Nataro JP. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

