Prolactin and male fertility: the long and short feedback regulation
- PMID: 20011060
- PMCID: PMC2778443
- DOI: 10.1155/2009/687259
Prolactin and male fertility: the long and short feedback regulation
Abstract
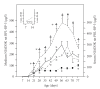
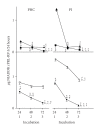
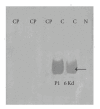
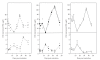
In the last 20 years, a pituitary-hypothalamus tissue culture system with intact neural and portal connections has been developed in our lab and used to understand the feedback mechanisms that regulate the secretions of adenohypophyseal hormones and fertility of male rats. In the last decade, several in vivo rat models have also been developed in our lab with a view to substantiate the in vitro findings, in order to delineate the role of pituitary hormones in the regulation of fertility of male rats. These studies have relied on both surgical and pharmacological interventions to modulate the secretions of gonadotropins and testosterone. The interrelationship between the circadian release of reproductive hormones has also been ascertained in normal men. Our studies suggest that testosterone regulates the secretion of prolactin through a long feedback mechanism, which appears to have been conserved from rats to humans. These studies have filled in a major lacuna pertaining to the role of prolactin in male reproductive physiology by demonstrating the interdependence between testosterone and prolactin. Systemic levels of prolactin play a deterministic role in the mechanism of chromatin condensation during spermiogenesis.
Figures



Similar articles
-
Role of prolactin in the regulation of sensitivity of the hypothalamic-pituitary system to steroid feedback.Adv Exp Med Biol. 1987;219:153-75. doi: 10.1007/978-1-4684-5395-9_8. Adv Exp Med Biol. 1987. PMID: 3324676 Review.
-
Serum prolactin, FSH, LH and testosterone before and after vasectomy in normal men.Arch Androl. 1982 Jun;8(4):311-3. doi: 10.3109/01485018208990216. Arch Androl. 1982. PMID: 6810776
-
An examination of blood steroid and gonadotropin concentrations in relation to fertility status and testicular function in men.Fertil Steril. 1982 Oct;38(4):465-70. doi: 10.1016/s0015-0282(16)46582-9. Fertil Steril. 1982. PMID: 6811340
-
Endocrinology of male infertility.Br Med Bull. 1979 May;35(2):187-92. doi: 10.1093/oxfordjournals.bmb.a071568. Br Med Bull. 1979. PMID: 387166 Review.
-
Effects of Ramadhan fast on male fertility.Arch Androl. 1986;16(2):161-6. doi: 10.3109/01485018608986937. Arch Androl. 1986. PMID: 3090956
Cited by
-
Nicotine alters male reproductive hormones in male albino rats: The role of cessation.J Hum Reprod Sci. 2013 Jan;6(1):40-4. doi: 10.4103/0974-1208.112380. J Hum Reprod Sci. 2013. PMID: 23869150 Free PMC article.
-
The maternal hormone in the male brain: Sexually dimorphic distribution of prolactin signalling in the mouse brain.PLoS One. 2018 Dec 20;13(12):e0208960. doi: 10.1371/journal.pone.0208960. eCollection 2018. PLoS One. 2018. PMID: 30571750 Free PMC article.
-
Effect of Polycyclic Aromatic Hydrocarbons Exposure on Sperm DNA in Idiopathic Male Infertility.J Health Pollut. 2019 Mar 14;9(21):190309. doi: 10.5696/2156-9614-9.21.190309. eCollection 2019 Mar. J Health Pollut. 2019. PMID: 30931169 Free PMC article.
-
Non-human primates as a translational model for the study of male reproductive health.Nat Rev Urol. 2025 Jul 7:10.1038/s41585-025-01062-2. doi: 10.1038/s41585-025-01062-2. Online ahead of print. Nat Rev Urol. 2025. PMID: 40624316 Review.
-
Putative molecular mechanism underlying sperm chromatin remodelling is regulated by reproductive hormones.Clin Epigenetics. 2012 Dec 17;4(1):23. doi: 10.1186/1868-7083-4-23. Clin Epigenetics. 2012. PMID: 23241214 Free PMC article.
References
-
- Singh J, O'Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136(12):5311–5321. - PubMed
-
- Bhasin S, Fielder T, Peacock N, Sod-Mariah UA, Swerdloff RS. Dissociating antifertility effects of GnRH-antagonist from its adverse effects on mating behavior in male rats. American Journal of Physiology, Endocrinology and Metabolism. 1988;254(1):E84–E91. - PubMed
-
- Mangurian LP, Walsh RJ, Posner BI. Prolactin enhancement of its own uptake at the choroids plexus. Endocrinology. 1992;131(2):698–702. - PubMed
-
- Grattan DR. The actions of prolactin in the brain during pregnancy and lactation. Progress in Brain Research. 2001;133:153–171. - PubMed
-
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocrine Reviews. 1998;19(3):225–268. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

