Molecular mechanisms of receptor-mediated endocytosis in the renal proximal tubular epithelium
- PMID: 20011067
- PMCID: PMC2789548
- DOI: 10.1155/2010/403272
Molecular mechanisms of receptor-mediated endocytosis in the renal proximal tubular epithelium
Abstract
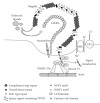
Receptor-mediated endocytosis is a pivotal function of renal proximal tubule epithelial cells (PTECs) to reabsorb and metabolize substantial amounts of proteins and other substances in glomerular filtrates. The function accounts for the conservation of nutrients, including carrier-bound vitamins and trace elements, filtered by glomeruli. Impairment of the process results in a loss of such substances and development of proteinuria, an important clinical sign of kidney disease and a risk marker for cardiovascular disease. Megalin is a multiligand endocytic receptor expressed at clathrin-coated pits of PTEC, playing a central role in the process. Megalin cooperates with various membrane molecules and interacts with many intracellular adaptor proteins for endocytic trafficking. Megalin is also involved in signaling pathways in the cells. Megalin-mediated endocytic overload leads to damage of PTEC. Further studies are needed to elucidate the mechanism of megalin-mediated endocytosis and develop strategies for preventing the damage of PTEC.
Figures
References
-
- Hjälm G, Murray E, Crumley G, et al. Cloning and sequencing of human gp330, a Ca2+-binding receptor with potential intracellular signaling properties. European Journal of Biochemistry. 1996;239(1):132–137. - PubMed
-
- Verroust PJ, Kozyraki R, Hammond TG, Moestrup SK, Christensen EI. Physiopathologic role of cubilin and megalin. Advances in Nephrology from the Necker Hospital. 2000;30:127–145. - PubMed
-
- Christensen EI, Verroust PJ, Nielsen R. Receptor-mediated endocytosis in renal proximal tubule. Pflügers Archiv European Journal of Physiology. 2009;458(6):1039–1048. - PubMed
-
- Davis CG, Goldstein JL, Sudhof TC, Anderson RG, Russell DW, Brown MS. Acid-dependent ligand dissociation and recycling of LDL receptor mediated by growth factor homology region. Nature. 1987;326(6115):760–765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

