Effects of triamcinolone acetonide on vessels of the posterior segment of the eye
- PMID: 20011077
- PMCID: PMC2791038
Effects of triamcinolone acetonide on vessels of the posterior segment of the eye
Abstract
Purpose: This study investigates the effects of triamcinolone acetonide (TA) on retinal endothelial cells in vitro and explores the potential vascular toxic effect of TA injected into the vitreous cavity of rats in vivo.
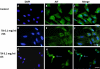
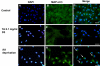
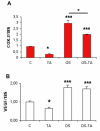
Methods: Subconfluent endothelial cells were treated with either 0.1 mg/ml or 1 mg/ml TA in 1% ethanol. Control cells were either untreated or exposed to 1% ethanol. Cell viability was evaluated at 24 h, 72 h, and five days using the tetrazolium 3-(4,5-dimethylthiazol-2-yl)-2,5 phenyltetrazolium bromide test (MTT) and lactate dehydrogenase (LDH) assays. Cell proliferation was evaluated by 5-bromo-2-deoxyuridine (BrdU) test. Apoptosis was evaluated by terminal deoxynucleotidyl transferase dUTP nick end labeling assay (TUNEL assay), annexin-binding, and caspase 3 activation. Caspase-independent cell deaths were investigated by immunohistochemistry using antibodies against apoptosis inducing factor (AIF), cytochrome C, microtubule-associated protein (MAP)-light chain 3 (MAP-LC3), and Leukocyte Elastase Inhibitor/Leukocyte Elastase Inhibitor-derived DNase II (LEI/L-DNase II). In vivo, semithin and ultrathin structure analysis and vascular casts were performed to examine TA-induced changes of the choroidal vasculature. In addition, outer segments phagocytosis assay on primary retinal pigment epithelium (RPE) cells was performed to assess cyclooxygenase (COX-2) and vascular endothelial growth factor (VEGF) mRNAs upregulation with or without TA.
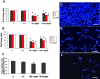
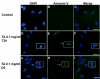
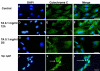
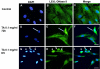
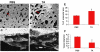
Results: The inhibitory effect of TA on cell proliferation could not explain the significant reduction in cell viability. Indeed, TA induced a time-dependent reduction of bovine retinal endothelial cells viability. Annexin-binding positive cells were observed. Cytochrome C was not released from mitochondria. L-DNase II was found translocated to the nucleus, meaning that LEI was changed into L-DNase II. AIF was found nuclearized in some cells. LC3 labeling showed the absence of autophagic vesicles. No autophagy or caspase dependent apoptosis was identified. At 1 mg/ml TA induced necrosis while exposure to lower concentrations for 3 to 5 days induced caspase independent apoptosis involving AIF and LEI/L-DNase II. In vivo, semithin and ultrathin structure analysis and vascular casts revealed that TA mostly affected the choroidal vasculature with a reduction of choroidal thickness and increased the avascular areas of the choriocapillaries. Experiments performed on primary RPE cells showed that TA downregulates the basal expression of COX-2 and VEGF and inhibits the outer segments (OS)-dependent COX-2 induction but not the OS-dependent VEGF induction.
Conclusions: This study demonstrates for the first time that glucocorticoids exert direct toxic effect on endothelial cells through caspase-independent cell death mechanisms. The choroidal changes observed after TA intravitreous injection may have important implications regarding the safety profile of TA use in human eyes.
Figures



Similar articles
-
Glucocorticoids induce retinal toxicity through mechanisms mainly associated with paraptosis.Mol Vis. 2007 Sep 19;13:1746-57. Mol Vis. 2007. PMID: 17960113
-
RETRACTED: Influence of choroidal neovascularization and biodegradable polymeric particle size on transscleral sustained delivery of triamcinolone acetonide.Int J Pharm. 2012 Sep 15;434(1-2):140-7. doi: 10.1016/j.ijpharm.2012.05.025. Epub 2012 May 23. Int J Pharm. 2012. PMID: 22633904 Free PMC article.
-
Triamcinolone reduces neovascularization, capillary density and IGF-1 receptor phosphorylation in a model of oxygen-induced retinopathy.Invest Ophthalmol Vis Sci. 2006 Nov;47(11):4975-82. doi: 10.1167/iovs.06-0450. Invest Ophthalmol Vis Sci. 2006. PMID: 17065516 Free PMC article.
-
Vascular endothelial growth factor reduced and connective tissue growth factor induced by triamcinolone in ARPE19 cells under oxidative stress.Invest Ophthalmol Vis Sci. 2005 Mar;46(3):1062-8. doi: 10.1167/iovs.04-0761. Invest Ophthalmol Vis Sci. 2005. PMID: 15728566
-
Retinal Inhibition of CCR3 Induces Retinal Cell Death in a Murine Model of Choroidal Neovascularization.PLoS One. 2016 Jun 16;11(6):e0157748. doi: 10.1371/journal.pone.0157748. eCollection 2016. PLoS One. 2016. PMID: 27309355 Free PMC article.
Cited by
-
Osmotic induction of cyclooxygenase-2 in RPE cells: Stimulation of inflammasome activation.Mol Vis. 2019 Jun 30;25:329-344. eCollection 2019. Mol Vis. 2019. PMID: 31341381 Free PMC article.
-
Intracellular delivery of dendrimer triamcinolone acetonide conjugates into microglial and human retinal pigment epithelial cells.Eur J Pharm Biopharm. 2015 Sep;95(Pt B):239-49. doi: 10.1016/j.ejpb.2015.02.013. Epub 2015 Feb 19. Eur J Pharm Biopharm. 2015. PMID: 25701805 Free PMC article.
-
Nonsteroid anti-inflammatory therapy suppresses the development of proliferative vitreoretinopathy more effectively than a steroid one.Int Ophthalmol. 2018 Aug;38(4):1365-1378. doi: 10.1007/s10792-017-0594-3. Epub 2017 Jun 21. Int Ophthalmol. 2018. PMID: 28639085
-
Compared antioxidant activity among corticosteroids on cultured retinal pigment epithelial cells.Graefes Arch Clin Exp Ophthalmol. 2016 Dec;254(12):2411-2416. doi: 10.1007/s00417-016-3519-3. Epub 2016 Oct 14. Graefes Arch Clin Exp Ophthalmol. 2016. PMID: 27743160
-
Vascular and Collagen Target: A Rational Approach to Hypertrophic Scar Management.Adv Wound Care (New Rochelle). 2023 Jan;12(1):38-55. doi: 10.1089/wound.2020.1348. Epub 2021 Aug 10. Adv Wound Care (New Rochelle). 2023. PMID: 34328823 Free PMC article. Review.
References
-
- Jonas JB, Kamppeter BA, Harder B, Vossmerbaeumer U, Sauder G, Spandau UH. Intravitreal triamcinolone acetonide for diabetic macular edema: a prospective, randomized study. J Ocul Pharmacol Ther. 2006;22:200–7. - PubMed
-
- Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina. 2000;20:244–50. - PubMed
-
- Challa JK, Gillies MC, Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal triamcinolone: 18 month follow up. Aust N Z J Ophthalmol. 1998;26:277–81. - PubMed
-
- Lee J, Freeman WR, Azen SP, Chung EJ, Koh HJ. Prospective, randomized clinical trial of intravitreal triamcinolone treatment of neovascular age-related macular degeneration: one-year results. Retina. 2007;27:1205–13. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
