CD14 signaling restrains chronic inflammation through induction of p38-MAPK/SOCS-dependent tolerance
- PMID: 20011115
- PMCID: PMC2781632
- DOI: 10.1371/journal.ppat.1000687
CD14 signaling restrains chronic inflammation through induction of p38-MAPK/SOCS-dependent tolerance
Abstract
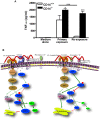
Current thinking emphasizes the primacy of CD14 in facilitating recognition of microbes by certain TLRs to initiate pro-inflammatory signaling events and the importance of p38-MAPK in augmenting such responses. Herein, this paradigm is challenged by demonstrating that recognition of live Borrelia burgdorferi not only triggers an inflammatory response in the absence of CD14, but one that is, in part, a consequence of altered PI3K/AKT/p38-MAPK signaling and impaired negative regulation of TLR2. CD14 deficiency results in increased localization of PI3K to lipid rafts, hyperphosphorylation of AKT, and reduced activation of p38. Such aberrant signaling leads to decreased negative regulation by SOCS1, SOCS3, and CIS, thereby compromising the induction of tolerance in macrophages and engendering more severe and persistent inflammatory responses to B. burgdorferi. Importantly, these altered signaling events and the higher cytokine production observed can be mimicked through shRNA and pharmacological inhibition of p38 activity in CD14-expressing macrophages. Perturbation of this CD14/p38-MAPK-dependent immune regulation may underlie development of infectious chronic inflammatory syndromes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Wooten RM, Weis JJ. Host-pathogen interactions promoting inflammatory Lyme arthritis: use of mouse models for dissection of disease processes. Curr Opin Microbiol. 2001;4:274–279. - PubMed
-
- Arbibe L, Mira JP, Teusch N, Kline L, Guha M, et al. Toll-like receptor 2-mediated NF-[kappa]B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. - PubMed
-
- Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–152. - PubMed
-
- Antal S. Evaluation of CD14 in host defence. European Journal of Clinical Investigation. 2000;30:167–179. - PubMed
-
- Sellati TJ, Bouis DA, Kitchens RL, Darveau RP, Pugin J, et al. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J Immunol. 1998;160:5455–5464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

