Network properties of robust immunity in plants
- PMID: 20011122
- PMCID: PMC2782137
- DOI: 10.1371/journal.pgen.1000772
Network properties of robust immunity in plants
Abstract
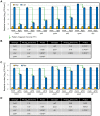
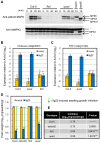
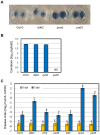
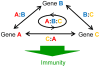
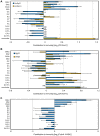
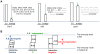
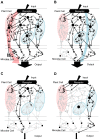
Two modes of plant immunity against biotrophic pathogens, Effector Triggered Immunity (ETI) and Pattern-Triggered Immunity (PTI), are triggered by recognition of pathogen effectors and Microbe-Associated Molecular Patterns (MAMPs), respectively. Although the jasmonic acid (JA)/ethylene (ET) and salicylic acid (SA) signaling sectors are generally antagonistic and important for immunity against necrotrophic and biotrophic pathogens, respectively, their precise roles and interactions in ETI and PTI have not been clear. We constructed an Arabidopsis dde2/ein2/pad4/sid2-quadruple mutant. DDE2, EIN2, and SID2 are essential components of the JA, ET, and SA sectors, respectively. The pad4 mutation affects the SA sector and a poorly characterized sector. Although the ETI triggered by the bacterial effector AvrRpt2 (AvrRpt2-ETI) and the PTI triggered by the bacterial MAMP flg22 (flg22-PTI) were largely intact in plants with mutations in any one of these genes, they were mostly abolished in the quadruple mutant. For the purposes of this study, AvrRpt2-ETI and flg22-PTI were measured as relative growth of Pseudomonas syringae bacteria within leaves. Immunity to the necrotrophic fungal pathogen Alternaria brassicicola was also severely compromised in the quadruple mutant. Quantitative measurements of the immunity levels in all combinatorial mutants and wild type allowed us to estimate the effects of the wild-type genes and their interactions on the immunity by fitting a mixed general linear model. This signaling allocation analysis showed that, contrary to current ideas, each of the JA, ET, and SA signaling sectors can positively contribute to immunity against both biotrophic and necrotrophic pathogens. The analysis also revealed that while flg22-PTI and AvrRpt2-ETI use a highly overlapping signaling network, the way they use the common network is very different: synergistic relationships among the signaling sectors are evident in PTI, which may amplify the signal; compensatory relationships among the sectors dominate in ETI, explaining the robustness of ETI against genetic and pathogenic perturbations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity.Curr Opin Plant Biol. 2010 Aug;13(4):459-65. doi: 10.1016/j.pbi.2010.04.006. Epub 2010 May 12. Curr Opin Plant Biol. 2010. PMID: 20471306 Review.
-
A plant effector-triggered immunity signaling sector is inhibited by pattern-triggered immunity.EMBO J. 2017 Sep 15;36(18):2758-2769. doi: 10.15252/embj.201796529. Epub 2017 Aug 15. EMBO J. 2017. PMID: 28811287 Free PMC article.
-
Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana.PLoS Genet. 2013;9(12):e1004015. doi: 10.1371/journal.pgen.1004015. Epub 2013 Dec 12. PLoS Genet. 2013. PMID: 24348271 Free PMC article.
-
Pattern-triggered immunity suppresses programmed cell death triggered by fumonisin b1.PLoS One. 2013;8(4):e60769. doi: 10.1371/journal.pone.0060769. Epub 2013 Apr 1. PLoS One. 2013. PMID: 23560104 Free PMC article.
-
Different Pathogen Defense Strategies in Arabidopsis: More than Pathogen Recognition.Cells. 2018 Dec 7;7(12):252. doi: 10.3390/cells7120252. Cells. 2018. PMID: 30544557 Free PMC article. Review.
Cited by
-
WRKY22 and WRKY25 transcription factors are positive regulators of defense responses in Nicotiana benthamiana.Plant Mol Biol. 2021 Jan;105(1-2):65-82. doi: 10.1007/s11103-020-01069-w. Epub 2020 Sep 9. Plant Mol Biol. 2021. PMID: 32909182
-
Layered pattern receptor signaling via ethylene and endogenous elicitor peptides during Arabidopsis immunity to bacterial infection.Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):6211-6. doi: 10.1073/pnas.1216780110. Epub 2013 Feb 19. Proc Natl Acad Sci U S A. 2013. PMID: 23431187 Free PMC article.
-
Wide screening of phage-displayed libraries identifies immune targets in planta.PLoS One. 2013;8(1):e54654. doi: 10.1371/journal.pone.0054654. Epub 2013 Jan 25. PLoS One. 2013. PMID: 23372747 Free PMC article.
-
Critical Role of COI1-Dependent Jasmonate Pathway in AAL toxin induced PCD in Tomato Revealed by Comparative Proteomics.Sci Rep. 2016 Jun 21;6:28451. doi: 10.1038/srep28451. Sci Rep. 2016. PMID: 27324416 Free PMC article.
-
Salicylic Acid Is Required for Broad-Spectrum Disease Resistance in Rice.Int J Mol Sci. 2022 Jan 25;23(3):1354. doi: 10.3390/ijms23031354. Int J Mol Sci. 2022. PMID: 35163275 Free PMC article.
References
-
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. - PubMed
-
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell. 2006;124:803–814. - PubMed
-
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, et al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. - PubMed
-
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, et al. Perception of the bacterial PAMP EF-tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

