Primate-specific origins and migration of cortical GABAergic neurons
- PMID: 20011218
- PMCID: PMC2790953
- DOI: 10.3389/neuro.05.026.2009
Primate-specific origins and migration of cortical GABAergic neurons
Abstract
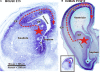
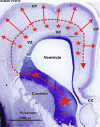
Gamma-aminobutyric-acidergic (GABAergic) cells form a very heterogeneous population of neurons that play a crucial role in the coordination and integration of cortical functions. Their number and diversity increase through mammalian brain evolution. Does evolution use the same or different developmental rules to provide the increased population of cortical GABAergic neurons? In rodents, these neurons are not generated in the pallial proliferative zones as glutamatergic principal neurons. They are produced almost exclusively by the subpallial proliferative zones, the ganglionic eminence (GE) and migrate tangentially to reach their target cortical layers. The GE is organized in molecularly different subdomains that produce different subpopulations of cortical GABAergic neurons. In humans and non-human primates, in addition to the GE, cortical GABAergic neurons are also abundantly generated by the proliferative zones of the dorsal telencephalon. Neurogenesis in ventral and dorsal telencephalon occurs with distinct temporal profiles. These dorsal and ventral lineages give rise to different populations of GABAergic neurons. Early-generated GABAergic neurons originate from the GE and mostly migrate to the marginal zone and the subplate. Later-generated GABAergic neurons, originating from both proliferative sites, populate the cortical plate. Interestingly, the pool of GABAergic progenitors in dorsal telencephalon produces mainly calretinin neurons, a population known to be significantly increased and to display specific features in primates. We conclude that the development of cortical GABAergic neurons have exclusive features in primates that need to be considered in order to understand pathological mechanisms leading to some neurological and psychiatric diseases.
Keywords: ganglionic eminence; glutamic acid decarboxylase; interneurons; neurogenesis; tangential migration; ventricular zone.
Figures





References
-
- Ascoli G. A., Alonso-Nanclares L., Anderson S. A., Barrionuevo G., Benavides-Piccione R., Burkhalter A., Buzsaki G., Cauli B., Defelipe J., Fairen A., Feldmeyer D., Fishell G., Fregnac Y., Freund T. F., Gardner D., Gardner E. P., Goldberg J. H., Helmstaedter M., Hestrin S., Karube F., Kisvarday Z. F., Lambolez B., Lewis D. A., Marin O., Markram H., Munoz A., Packer A., Petersen C. C., Rockland K. S., Rossier J., Rudy B., Somogyi P., Staiger J. F., Tamas G., Thomson A. M., Toledo-Rodriguez M., Wang Y., West D. C., Yuste R. (2008). Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 9, 557–568 10.1038/nrn2402 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

