The 3'-phosphoadenosine 5'-phosphosulfate transporters, PAPST1 and 2, contribute to the maintenance and differentiation of mouse embryonic stem cells
- PMID: 20011239
- PMCID: PMC2788424
- DOI: 10.1371/journal.pone.0008262
The 3'-phosphoadenosine 5'-phosphosulfate transporters, PAPST1 and 2, contribute to the maintenance and differentiation of mouse embryonic stem cells
Abstract
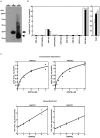
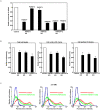
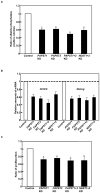
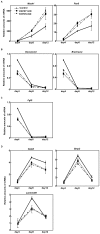
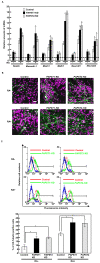
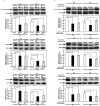
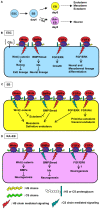
Recently, we have identified two 3'-phosphoadenosine 5'-phosphosulfate (PAPS) transporters (PAPST1 and PAPST2), which contribute to PAPS transport into the Golgi, in both human and Drosophila. Mutation and RNA interference (RNAi) of the Drosophila PAPST have shown the importance of PAPST-dependent sulfation of carbohydrates and proteins during development. However, the functional roles of PAPST in mammals are largely unknown. Here, we investigated whether PAPST-dependent sulfation is involved in regulating signaling pathways required for the maintenance of mouse embryonic stem cells (mESCs), differentiation into the three germ layers, and neurogenesis. By using a yeast expression system, mouse PAPST1 and PAPST2 proteins were shown to have PAPS transport activity with an apparent K(m) value of 1.54 microM or 1.49 microM, respectively. RNAi-mediated knockdown of each PAPST induced the reduction of chondroitin sulfate (CS) chain sulfation as well as heparan sulfate (HS) chain sulfation, and inhibited mESC self-renewal due to defects in several signaling pathways. However, we suggest that these effects were due to reduced HS, not CS, chain sulfation, because knockdown of mouse N-deacetylase/N-sulfotransferase, which catalyzes the first step of HS sulfation, in mESCs gave similar results to those observed in PAPST-knockdown mESCs, but depletion of CS chains did not. On the other hand, during embryoid body formation, PAPST-knockdown mESCs exhibited abnormal differentiation, in particular neurogenesis was promoted, presumably due to the observed defects in BMP, FGF and Wnt signaling. The latter were reduced as a result of the reduction in both HS and CS chain sulfation. We propose that PAPST-dependent sulfation of HS or CS chains, which is regulated developmentally, regulates the extrinsic signaling required for the maintenance and normal differentiation of mESCs.
Conflict of interest statement
Figures

References
-
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
-
- Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. - PubMed
-
- Chambers I, Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004;23:7150–7160. - PubMed
-
- Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, et al. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

