Discrimination of carcinogens by hepatic transcript profiling in rats following 28-day administration
- PMID: 20011461
- PMCID: PMC2791490
- DOI: 10.4137/cin.s3229
Discrimination of carcinogens by hepatic transcript profiling in rats following 28-day administration
Abstract
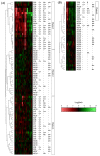
This study aimed at discriminating carcinogens on the basis of hepatic transcript profiling in the rats administrated with a variety of carcinogens and non-carcinogens. We conducted 28-day toxicity tests in male F344 rats with 47 carcinogens and 26 non-carcinogens, and then investigated periodically the hepatic gene expression profiles using custom microarrays. By hierarchical cluster analysis based on significantly altered genes, carcinogens were clustered into three major groups (Group 1 to 3). The formation of these groups was not affected by the gene sets used as well as the administration period, indicating that the grouping of carcinogens was universal independent of the conditions of both statistical analysis and toxicity testing. Seventeen carcinogens belonging to Group 1 were composed of mainly rat hepatocarcinogens, most of them being mutagenic ones. Group 2 was formed by three subgroups, which were composed of 23 carcinogens exhibiting distinct properties in terms of genotoxicity and target tissues, namely nonmutagenic hepatocarcinogens, and mutagenic and nonmutagenic carcinogens both of which are targeted to other tissues. Group 3 contained 6 carcinogens including 4 estrogenic substances, implying the group of estrogenic carcinogens. Gene network analyses revealed that the significantly altered genes in Group 1 included Bax, Tnfrsf6, Btg2, Mgmt and Abcb1b, suggesting that p53-mediated signaling pathway involved in early pathologic alterations associated with preceding mutagenic carcinogenesis. Thus, the common transcriptional signatures for each group might reflect the early molecular events of carcinogenesis and hence would enable us to identify the biomarker genes, and then to develop a new assay for carcinogenesis prediction.
Keywords: carcinogenicity; cluster analysis; hepatocarcinogen; microarray; mutagenicity; toxicogenomics.
Figures


References
-
- Usui T, Mutai M, Hisada S, et al. CB6F1-rasH2 mouse: overview of available data. Toxicol Pathol. 2001;29(Suppl):90–108. - PubMed
-
- Wetmore BA, Merrick BA. Toxicoproteomics: proteomics applied to toxicology and pathology. Toxicol Pathol. 2004;32:619–42. - PubMed
-
- Tennant RW, Margolin BH, Shelby MD, et al. Prediction of chemical carcinogenicity in rodents from in vitro genetic toxicity assays. Science. 1987 May 22;236(4804):933–41. - PubMed
-
- Tennant RW. Relationships between in vitro genetic toxicity and carcinogenicity studies in animals. Ann N Y Acad Sci. 1988;534:127–32. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

