Hypoxia and the hypoxic response pathway protect against pore-forming toxins in C. elegans
- PMID: 20011506
- PMCID: PMC2785477
- DOI: 10.1371/journal.ppat.1000689
Hypoxia and the hypoxic response pathway protect against pore-forming toxins in C. elegans
Abstract
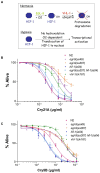
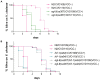
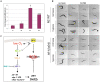
Pore-forming toxins (PFTs) are by far the most abundant bacterial protein toxins and are important for the virulence of many important pathogens. As such, cellular responses to PFTs critically modulate host-pathogen interactions. Although many cellular responses to PFTs have been recorded, little is understood about their relevance to pathological or defensive outcomes. To shed light on this important question, we have turned to the only genetic system for studying PFT-host interactions-Caenorhabditis elegans intoxication by Crystal (Cry) protein PFTs. We mutagenized and screened for C. elegans mutants resistant to a Cry PFT and recovered one mutant. Complementation, sequencing, transgenic rescue, and RNA interference data demonstrate that this mutant eliminates a gene normally involved in repression of the hypoxia (low oxygen response) pathway. We find that up-regulation of the C. elegans hypoxia pathway via the inactivation of three different genes that normally repress the pathway results in animals resistant to Cry PFTs. Conversely, mutation in the central activator of the hypoxia response, HIF-1, suppresses this resistance and can result in animals defective in PFT defenses. These results extend to a PFT that attacks mammals since up-regulation of the hypoxia pathway confers resistance to Vibrio cholerae cytolysin (VCC), whereas down-regulation confers hypersusceptibility. The hypoxia PFT defense pathway acts cell autonomously to protect the cells directly under attack and is different from other hypoxia pathway stress responses. Two of the downstream effectors of this pathway include the nuclear receptor nhr-57 and the unfolded protein response. In addition, the hypoxia pathway itself is induced by PFT, and low oxygen is protective against PFT intoxication. These results demonstrate that hypoxia and induction of the hypoxia response protect cells against PFTs, and that the cellular environment can be modulated via the hypoxia pathway to protect against the most prevalent class of weapons used by pathogenic bacteria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Global functional analyses of cellular responses to pore-forming toxins.PLoS Pathog. 2011 Mar;7(3):e1001314. doi: 10.1371/journal.ppat.1001314. Epub 2011 Mar 3. PLoS Pathog. 2011. PMID: 21408619 Free PMC article.
-
Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo.PLoS Pathog. 2008 Oct;4(10):e1000176. doi: 10.1371/journal.ppat.1000176. Epub 2008 Oct 10. PLoS Pathog. 2008. PMID: 18846208 Free PMC article.
-
Genomic analysis of immune response against Vibrio cholerae hemolysin in Caenorhabditis elegans.PLoS One. 2012;7(5):e38200. doi: 10.1371/journal.pone.0038200. Epub 2012 May 31. PLoS One. 2012. PMID: 22675448 Free PMC article.
-
Pore worms: using Caenorhabditis elegans to study how bacterial toxins interact with their target host.Int J Med Microbiol. 2004 Apr;293(7-8):599-607. doi: 10.1078/1438-4221-00303. Int J Med Microbiol. 2004. PMID: 15149037 Review.
-
X-ray crystallography shines a light on pore-forming toxins.Methods Enzymol. 2021;649:1-46. doi: 10.1016/bs.mie.2021.01.001. Epub 2021 Feb 10. Methods Enzymol. 2021. PMID: 33712183 Review.
Cited by
-
HIF-1 modulates longevity and healthspan in a temperature-dependent manner.Aging Cell. 2011 Apr;10(2):318-26. doi: 10.1111/j.1474-9726.2011.00672.x. Epub 2011 Feb 23. Aging Cell. 2011. PMID: 21241450 Free PMC article.
-
Monalysin, a novel ß-pore-forming toxin from the Drosophila pathogen Pseudomonas entomophila, contributes to host intestinal damage and lethality.PLoS Pathog. 2011 Sep;7(9):e1002259. doi: 10.1371/journal.ppat.1002259. Epub 2011 Sep 29. PLoS Pathog. 2011. PMID: 21980286 Free PMC article.
-
Transcriptome analyses describe the consequences of persistent HIF-1 over-activation in Caenorhabditis elegans.PLoS One. 2024 Mar 22;19(3):e0295093. doi: 10.1371/journal.pone.0295093. eCollection 2024. PLoS One. 2024. PMID: 38517909 Free PMC article.
-
PRMT-7/PRMT7 activates HLH-30/TFEB to guard plasma membrane integrity compromised by bacterial pore-forming toxins.Autophagy. 2024 Jun;20(6):1335-1358. doi: 10.1080/15548627.2024.2306655. Epub 2024 Feb 19. Autophagy. 2024. PMID: 38261662 Free PMC article.
-
The hypoxia-inducible factor HIF-1 functions as both a positive and negative modulator of aging.Biol Chem. 2010 Oct;391(10):1131-7. doi: 10.1515/BC.2010.123. Biol Chem. 2010. PMID: 20707608 Free PMC article. Review.
References
-
- Alouf JE, Popoff MR. The comprehensive sourcebook of bacterial protein toxins. London: Academic Press; 2005.
-
- Parker MW, Feil SC. Pore-forming protein toxins: from structure to function. Prog Biophys Mol Biol. 2005;88:91–142. - PubMed
-
- Aroian R, van der Goot FG. Pore-forming toxins and cellular non-immune defenses (CNIDs). Curr Opin Microbiol. 2007;10:57–61. - PubMed
-
- Husmann M, Beckmann E, Boller K, Kloft N, Tenzer S, et al. Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett. 2009;583:337–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

