Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells
- PMID: 20011520
- PMCID: PMC2788414
- DOI: 10.1371/journal.pone.0008248
Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells
Abstract
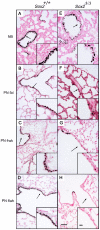
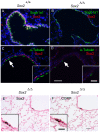
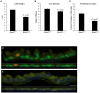
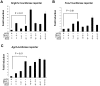
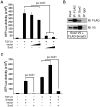
The bronchioles of the murine lung are lined by a simple columnar epithelium composed of ciliated, Clara, and goblet cells that together mediate barrier function, mucociliary clearance and innate host defense, vital for pulmonary homeostasis. In the present work, we demonstrate that expression of Sox2 in Clara cells is required for the differentiation of ciliated, Clara, and goblet cells that line the bronchioles of the postnatal lung. The gene was selectively deleted in Clara cells utilizing Scgb1a1-Cre, causing the progressive loss of Sox2 in the bronchioles during perinatal and postnatal development. The rate of bronchiolar cell proliferation was decreased and associated with the formation of an undifferentiated, cuboidal-squamous epithelium lacking the expression of markers of Clara cells (Scgb1a1), ciliated cells (FoxJ1 and alpha-tubulin), and goblet cells (Spdef and Muc5AC). By adulthood, bronchiolar cell numbers were decreased and Sox2 was absent in extensive regions of the bronchiolar epithelium, at which time residual Sox2 expression was primarily restricted to selective niches of CGRP staining neuroepithelial cells. Allergen-induced goblet cell differentiation and mucus production was absent in the respiratory epithelium lacking Sox2. In vitro, Sox2 activated promoter-luciferase reporter constructs for differentiation markers characteristic of Clara, ciliated, and goblet cells, Scgb1a1, FoxJ1, and Agr2, respectively. Sox2 physically interacted with Smad3 and inhibited TGF-beta1/Smad3-mediated transcriptional activity in vitro, a pathway that negatively regulates proliferation. Sox2 is required for proliferation and differentiation of Clara cells that serve as the progenitor cells from which Clara, ciliated, and goblet cells are derived.
Conflict of interest statement
Figures


Similar articles
-
Foxm1 transcription factor is critical for proliferation and differentiation of Clara cells during development of conducting airways.Dev Biol. 2012 Oct 15;370(2):198-212. doi: 10.1016/j.ydbio.2012.07.028. Epub 2012 Aug 2. Dev Biol. 2012. PMID: 22885335 Free PMC article.
-
SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production.J Clin Invest. 2009 Oct;119(10):2914-24. doi: 10.1172/JCI39731. Epub 2009 Sep 14. J Clin Invest. 2009. PMID: 19759516 Free PMC article.
-
Transdifferentiation of ciliated cells during repair of the respiratory epithelium.Am J Respir Cell Mol Biol. 2006 Feb;34(2):151-7. doi: 10.1165/rcmb.2005-0332OC. Epub 2005 Oct 20. Am J Respir Cell Mol Biol. 2006. PMID: 16239640 Free PMC article.
-
Clara cell: progenitor for the bronchiolar epithelium.Int J Biochem Cell Biol. 2010 Jan;42(1):1-4. doi: 10.1016/j.biocel.2009.09.002. Epub 2009 Sep 9. Int J Biochem Cell Biol. 2010. PMID: 19747565 Free PMC article. Review.
-
Regulation of mucin expression in respiratory diseases.Biochem Soc Trans. 2009 Aug;37(Pt 4):877-81. doi: 10.1042/BST0370877. Biochem Soc Trans. 2009. PMID: 19614611 Review.
Cited by
-
Consequences of telomere dysfunction in fibroblasts, club and basal cells for lung fibrosis development.Nat Commun. 2022 Oct 6;13(1):5656. doi: 10.1038/s41467-022-32771-6. Nat Commun. 2022. PMID: 36202783 Free PMC article.
-
Notch Transduction in Non-Small Cell Lung Cancer.Int J Mol Sci. 2020 Aug 8;21(16):5691. doi: 10.3390/ijms21165691. Int J Mol Sci. 2020. PMID: 32784481 Free PMC article. Review.
-
Understanding Lung Carcinogenesis from a Morphostatic Perspective: Prevention and Therapeutic Potential of Phytochemicals for Targeting Cancer Stem Cells.Int J Mol Sci. 2021 May 27;22(11):5697. doi: 10.3390/ijms22115697. Int J Mol Sci. 2021. PMID: 34071790 Free PMC article. Review.
-
Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer.Nat Genet. 2012 Oct;44(10):1111-6. doi: 10.1038/ng.2405. Epub 2012 Sep 2. Nat Genet. 2012. PMID: 22941189 Free PMC article.
-
A new mouse model to study the role of ectopic Nanos3 expression in cancer.BMC Cancer. 2019 Jun 17;19(1):598. doi: 10.1186/s12885-019-5807-x. BMC Cancer. 2019. PMID: 31208373 Free PMC article.
References
-
- Evans MJ, Cabral LC, Stephens RJ, Freeman G. Acute kinetic response and renewal of the alveolar epithelium following injury by nitrogen dioxide. Chest. 1974;65:(Suppl):62S–65S. - PubMed
-
- Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22:142–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

