N-cadherin negatively regulates osteoblast proliferation and survival by antagonizing Wnt, ERK and PI3K/Akt signalling
- PMID: 20011526
- PMCID: PMC2788421
- DOI: 10.1371/journal.pone.0008284
N-cadherin negatively regulates osteoblast proliferation and survival by antagonizing Wnt, ERK and PI3K/Akt signalling
Abstract
Background: Osteoblasts are bone forming cells that play an essential role in osteogenesis. The elucidation of the mechanisms that control osteoblast number is of major interest for the treatment of skeletal disorders characterized by abnormal bone formation. Canonical Wnt signalling plays an important role in the control of osteoblast proliferation, differentiation and survival. Recent studies indicate that the cell-cell adhesion molecule N-cadherin interacts with the Wnt co-receptors LRP5/6 to regulate osteoblast differentiation and bone accrual. The role of N-cadherin in the control of osteoblast proliferation and survival remains unknown.
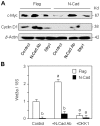
Methods and principal findings: Using murine MC3T3-E1 osteoblastic cells and N-cadherin transgenic mice, we demonstrate that N-cadherin overexpression inhibits cell proliferation in vitro and in vivo. The negative effect of N-cadherin on cell proliferation results from decreased Wnt, ERK and PI3K/Akt signalling and is restored by N-cadherin neutralizing antibody that antagonizes N-cadherin-LRP5 interaction. Inhibition of Wnt signalling using DKK1 or Sfrp1 abolishes the ability of N-cadherin blockade to restore ERK and PI3K signalling and cell proliferation, indicating that the altered cell growth in N-cadherin overexpressing cells is in part secondary to alterations in Wnt signalling. Consistently, we found that N-cadherin overexpression inhibits the expression of Wnt3a ligand and its downstream targets c-myc and cyclin D1, an effect that is partially reversed by N-cadherin blockade. We also show that N-cadherin overexpression decreases osteoblast survival in vitro and in vivo. This negative effect on cell survival results from inhibition of PI3K/Akt signalling and increased Bax/Bcl-2, a mechanism that is rescued by Wnt3a.
Conclusion: The data show that N-cadherin negatively controls osteoblast proliferation and survival via inhibition of autocrine/paracrine Wnt3a ligand expression and attenuation of Wnt, ERK and PI3K/Akt signalling, which provides novel mechanisms by which N-cadherin regulates osteoblast number.
Conflict of interest statement
Figures







Similar articles
-
N-cadherin interacts with axin and LRP5 to negatively regulate Wnt/beta-catenin signaling, osteoblast function, and bone formation.Mol Cell Biol. 2009 Feb;29(4):953-64. doi: 10.1128/MCB.00349-08. Epub 2008 Dec 15. Mol Cell Biol. 2009. PMID: 19075000 Free PMC article.
-
Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT.J Biol Chem. 2005 Dec 16;280(50):41342-51. doi: 10.1074/jbc.M502168200. Epub 2005 Oct 25. J Biol Chem. 2005. PMID: 16251184
-
Receptor for advanced glycation end products inhibits proliferation in osteoblast through suppression of Wnt, PI3K and ERK signaling.Biochem Biophys Res Commun. 2012 Jul 13;423(4):684-9. doi: 10.1016/j.bbrc.2012.06.015. Epub 2012 Jun 12. Biochem Biophys Res Commun. 2012. PMID: 22699121
-
Role of N-cadherin in bone formation.J Cell Physiol. 2002 Mar;190(3):297-305. doi: 10.1002/jcp.10073. J Cell Physiol. 2002. PMID: 11857445 Review.
-
Wnt signaling control of bone cell apoptosis.Cell Res. 2008 Feb;18(2):248-53. doi: 10.1038/cr.2008.13. Cell Res. 2008. PMID: 18212734 Review.
Cited by
-
Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/β-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways.Neoplasia. 2011 Dec;13(12):1101-12. doi: 10.1593/neo.111060. Neoplasia. 2011. PMID: 22247700 Free PMC article.
-
Wnt/beta-catenin signaling and small molecule inhibitors.Curr Pharm Des. 2013;19(4):634-64. doi: 10.2174/138161213804581837. Curr Pharm Des. 2013. PMID: 23016862 Free PMC article. Review.
-
Cadherin-mediated cell-cell adhesion and signaling in the skeleton.Calcif Tissue Int. 2014 Jan;94(1):46-54. doi: 10.1007/s00223-013-9733-7. Epub 2013 May 9. Calcif Tissue Int. 2014. PMID: 23657489 Free PMC article. Review.
-
Role of Neural (N)-Cadherin in Breast Cancer Cell Stemness and Dormancy in the Bone Microenvironment.Cancers (Basel). 2022 Mar 4;14(5):1317. doi: 10.3390/cancers14051317. Cancers (Basel). 2022. PMID: 35267624 Free PMC article.
-
p85alpha regulates osteoblast differentiation by cross-talking with the MAPK pathway.J Biol Chem. 2011 Apr 15;286(15):13512-21. doi: 10.1074/jbc.M110.187351. Epub 2011 Feb 15. J Biol Chem. 2011. PMID: 21324896 Free PMC article.
References
-
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. - PubMed
-
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. - PubMed
-
- Nusse R, Fuerer C, Ching W, Harnish K, Logan C, et al. Wnt signaling and stem cell control. Cold Spring Harb Symp Quant Biol. 2008;73:59–66. - PubMed
-
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

