Activin signaling in microsatellite stable colon cancers is disrupted by a combination of genetic and epigenetic mechanisms
- PMID: 20011542
- PMCID: PMC2789408
- DOI: 10.1371/journal.pone.0008308
Activin signaling in microsatellite stable colon cancers is disrupted by a combination of genetic and epigenetic mechanisms
Abstract
Background: Activin receptor 2 (ACVR2) is commonly mutated in microsatellite unstable (MSI) colon cancers, leading to protein loss, signaling disruption, and larger tumors. Here, we examined activin signaling disruption in microsatellite stable (MSS) colon cancers.
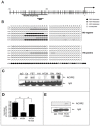
Methods: Fifty-one population-based MSS colon cancers were assessed for ACVR1, ACVR2 and pSMAD2 protein. Consensus mutation-prone portions of ACVR2 were sequenced in primary cancers and all exons in colon cancer cell lines. Loss of heterozygosity (LOH) was evaluated for ACVR2 and ACVR1, and ACVR2 promoter methylation by methylation-specific PCR and bisulfite sequencing and chromosomal instability (CIN) phenotype via fluorescent LOH analysis of 3 duplicate markers. ACVR2 promoter methylation and ACVR2 expression were assessed in colon cancer cell lines via qPCR and IP-Western blots. Re-expression of ACVR2 after demethylation with 5-aza-2'-deoxycytidine (5-Aza) was determined. An additional 26 MSS colon cancers were assessed for ACVR2 loss and its mechanism, and ACVR2 loss in all tested cancers correlated with clinicopathological criteria.
Results: Of 51 MSS colon tumors, 7 (14%) lost ACVR2, 2 (4%) ACVR1, and 5 (10%) pSMAD2 expression. No somatic ACVR2 mutations were detected. Loss of ACVR2 expression was associated with LOH at ACVR2 (p<0.001) and ACVR2 promoter hypermethylation (p<0.05). ACVR2 LOH, but not promoter hypermethylation, correlated with CIN status. In colon cancer cell lines with fully methylated ACVR2 promoter, loss of ACVR2 mRNA and protein expression was restored with 5-Aza treatment. Loss of ACVR2 was associated with an increase in primary colon cancer volume (p<0.05).
Conclusions: Only a small percentage of MSS colon cancers lose expression of activin signaling members. ACVR2 loss occurs through LOH and ACVR2 promoter hypermethylation, revealing distinct mechanisms for ACVR2 inactivation in both MSI and MSS subtypes of colon cancer.
Conflict of interest statement
Figures


Similar articles
-
Loss of activin receptor type 2 protein expression in microsatellite unstable colon cancers.Gastroenterology. 2004 Mar;126(3):654-9. doi: 10.1053/j.gastro.2004.01.008. Gastroenterology. 2004. PMID: 14988818
-
Activin type 2 receptor restoration in MSI-H colon cancer suppresses growth and enhances migration with activin.Gastroenterology. 2007 Feb;132(2):633-44. doi: 10.1053/j.gastro.2006.11.018. Epub 2006 Nov 16. Gastroenterology. 2007. PMID: 17258738 Free PMC article.
-
Activin type II receptor restoration in ACVR2-deficient colon cancer cells induces transforming growth factor-beta response pathway genes.Cancer Res. 2004 Nov 1;64(21):7690-6. doi: 10.1158/0008-5472.CAN-04-2082. Cancer Res. 2004. PMID: 15520171
-
Epigenetic inactivation of RUNX3 in microsatellite unstable sporadic colon cancers.Int J Cancer. 2004 Dec 10;112(5):754-9. doi: 10.1002/ijc.20472. Int J Cancer. 2004. PMID: 15386381
-
Genetic pathways and mutation profiles of human cancers: site- and exposure-specific patterns.Carcinogenesis. 2007 Sep;28(9):1851-8. doi: 10.1093/carcin/bgm176. Epub 2007 Aug 11. Carcinogenesis. 2007. PMID: 17693665 Free PMC article. Review.
Cited by
-
Transforming Growth Factor β Superfamily Signaling in Development of Colorectal Cancer.Gastroenterology. 2017 Jan;152(1):36-52. doi: 10.1053/j.gastro.2016.10.015. Epub 2016 Oct 20. Gastroenterology. 2017. PMID: 27773809 Free PMC article. Review.
-
Does the expression of the ACVR2A gene affect the development of colorectal cancer?Genet Mol Biol. 2019 Jan-Mar;42(1):32-39. doi: 10.1590/1678-4685-GMB-2017-0332. Epub 2019 Mar 11. Genet Mol Biol. 2019. PMID: 30856244 Free PMC article.
-
Instability of a dinucleotide repeat in the 3'-untranslated region (UTR) of the microsomal prostaglandin E synthase-1 (mPGES-1) gene in microsatellite instability-high (MSI-H) colorectal carcinoma.Mol Oncol. 2015 Aug;9(7):1252-8. doi: 10.1016/j.molonc.2015.01.009. Epub 2015 Mar 5. Mol Oncol. 2015. PMID: 25817443 Free PMC article.
-
Immune genes are associated with human glioblastoma pathology and patient survival.BMC Med Genomics. 2012 Sep 14;5:41. doi: 10.1186/1755-8794-5-41. BMC Med Genomics. 2012. PMID: 22980038 Free PMC article.
-
Genetics and Genetic Biomarkers in Sporadic Colorectal Cancer.Gastroenterology. 2015 Oct;149(5):1177-1190.e3. doi: 10.1053/j.gastro.2015.06.047. Epub 2015 Jul 26. Gastroenterology. 2015. PMID: 26216840 Free PMC article. Review.
References
-
- Carethers JM. Rustgi A, Crawford J,, editors. Biology of Colorectal Carcinoma. , editors. . Gastrointestinal Cancers: Biology and Clinical Management. 2nd ed: WB Saunders. 2003. pp. 407–419.
-
- Jung B, Doctolero RT, Tajima A, Nguyen AK, Keku T, et al. Loss of activin receptor type 2 protein expression in microsatellite unstable colon cancers. Gastroenterology. 2004;126:654–659. - PubMed
-
- Grady WM, Myeroff LL, Swinler SE, Rajput A, Thiagalingam S, et al. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res. 1999;59:320–324. - PubMed
-
- Eppert K, Scherer SW, Ozcelik H, Pirone R, Hoodless P, et al. MADR2 maps to 18q21 and encodes a TGFbeta-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell. 1996;86:543–552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

