Impact of previous virological treatment failures and adherence on the outcome of antiretroviral therapy in 2007
- PMID: 20011544
- PMCID: PMC2789943
- DOI: 10.1371/journal.pone.0008275
Impact of previous virological treatment failures and adherence on the outcome of antiretroviral therapy in 2007
Abstract
Background: Combination antiretroviral treatment (cART) has been very successful, especially among selected patients in clinical trials. The aim of this study was to describe outcomes of cART on the population level in a large national cohort.
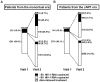
Methods: Characteristics of participants of the Swiss HIV Cohort Study on stable cART at two semiannual visits in 2007 were analyzed with respect to era of treatment initiation, number of previous virologically failed regimens and self reported adherence. Starting ART in the mono/dual era before HIV-1 RNA assays became available was counted as one failed regimen. Logistic regression was used to identify risk factors for virological failure between the two consecutive visits.
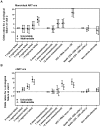
Results: Of 4541 patients 31.2% and 68.8% had initiated therapy in the mono/dual and cART era, respectively, and been on treatment for a median of 11.7 vs. 5.7 years. At visit 1 in 2007, the mean number of previous failed regimens was 3.2 vs. 0.5 and the viral load was undetectable (<50 copies/ml) in 84.6% vs. 89.1% of the participants, respectively. Adjusted odds ratios of a detectable viral load at visit 2 for participants from the mono/dual era with a history of 2 and 3, 4, >4 previous failures compared to 1 were 0.9 (95% CI 0.4-1.7), 0.8 (0.4-1.6), 1.6 (0.8-3.2), 3.3 (1.7-6.6) respectively, and 2.3 (1.1-4.8) for >2 missed cART doses during the last month, compared to perfect adherence. From the cART era, odds ratios with a history of 1, 2 and >2 previous failures compared to none were 1.8 (95% CI 1.3-2.5), 2.8 (1.7-4.5) and 7.8 (4.5-13.5), respectively, and 2.8 (1.6-4.8) for >2 missed cART doses during the last month, compared to perfect adherence.
Conclusions: A higher number of previous virologically failed regimens, and imperfect adherence to therapy were independent predictors of imminent virological failure.
Conflict of interest statement
Figures


References
-
- The Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study group. Response to combination antiretroviral therapy: variation by age. AIDS. 2008;22:1463–1473. - PubMed
-
- Bartlett JA, Chen SS, Quinn JB. Comparative efficacy of nucleoside/nucleotide reverse transcriptase inhibitors in combination with efavirenz: results of a systematic overview. HIV Clin Trials. 2007;8:221–226. - PubMed
-
- Eron J,, Jr., Yeni P, Gathe J,, Jr., Estrada V, DeJesus E, et al. The KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: a randomised non-inferiority trial. Lancet. 2006;368:476–482. - PubMed
-
- Cooper D, Gatell J, Rockstroh J, Katlama C, Yeni P, et al. 48-week results from BENCHMRK-1, a phase III study of raltegravir in patients failing ART with triple-class resistant HIV-1. 15th Conference on Retroviruses and Opportunistic Infections, February 3–6, Boston Ma, Abstract #. 2008;788

