Estradiol stimulates vasodilatory and metabolic pathways in cultured human endothelial cells
- PMID: 20011585
- PMCID: PMC2785884
- DOI: 10.1371/journal.pone.0008242
Estradiol stimulates vasodilatory and metabolic pathways in cultured human endothelial cells
Abstract
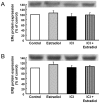
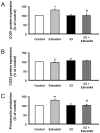
Vascular effects of estradiol are being investigated because there are controversies among clinical and experimental studies. DNA microarrays were used to investigate global gene expression patterns in cultured human umbilical vein endothelial cells (HUVEC) exposed to 1 nmol/L estradiol for 24 hours. When compared to control, 187 genes were identified as differentially expressed with 1.9-fold change threshold. Supervised principal component analysis and hierarchical cluster analysis revealed the differences between control and estradiol-treated samples. Physiological concentrations of estradiol are sufficient to elicit significant changes in HUVEC gene expression. Notch signaling, actin cytoskeleton signaling, pentose phosphate pathway, axonal guidance signaling and integrin signaling were the top-five canonical pathways significantly regulated by estrogen. A total of 26 regulatory networks were identified as estrogen responsive. Microarray data were confirmed by quantitative RT-PCR in cardiovascular meaning genes; cyclooxygenase (COX)1, dimethylarginine dimethylaminohydrolase (DDAH)2, phospholipase A2 group IV (PLA2G4) B, and 7-dehydrocholesterol reductase were up-regulated by estradiol in a dose-dependent and estrogen receptor-dependent way, whereas COX2, DDAH1 and PLA2G4A remained unaltered. Moreover, estradiol-induced COX1 gene expression resulted in increased COX1 protein content and enhanced prostacyclin production. DDAH2 protein content was also increased, which in turn decreased asymmetric dimethylarginine concentration and increased NO release. All stimulated effects of estradiol on gene and protein expression were estrogen receptor-dependent, since were abolished in the presence of the estrogen receptor antagonist ICI 182780. This study identifies new vascular mechanisms of action by which estradiol may contribute to a wide range of biological processes.
Conflict of interest statement
Figures






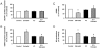
Similar articles
-
Estradiol selectively stimulates endothelial prostacyclin production through estrogen receptor-{alpha}.J Mol Endocrinol. 2010 Apr;44(4):237-46. doi: 10.1677/JME-09-0112. Epub 2010 Jan 28. J Mol Endocrinol. 2010. PMID: 20110403
-
Estradiol counteracts oxidized LDL-induced asymmetric dimethylarginine production by cultured human endothelial cells.Cardiovasc Res. 2007 Jan 1;73(1):66-72. doi: 10.1016/j.cardiores.2006.09.020. Epub 2006 Sep 30. Cardiovasc Res. 2007. PMID: 17097077
-
Estradiol-17β and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites selectively stimulate production of prostacyclin in uterine artery endothelial cells: role of estrogen receptor-α versus estrogen receptor-β.Hypertension. 2013 Feb;61(2):509-18. doi: 10.1161/HYPERTENSIONAHA.112.200717. Epub 2013 Jan 14. Hypertension. 2013. PMID: 23319543 Free PMC article.
-
Estradiol, acting through estrogen receptor alpha, restores dimethylarginine dimethylaminohydrolase activity and nitric oxide production in oxLDL-treated human arterial endothelial cells.Mol Cell Endocrinol. 2013 Jan 5;365(1):11-6. doi: 10.1016/j.mce.2012.08.020. Epub 2012 Sep 7. Mol Cell Endocrinol. 2013. PMID: 22982060
-
Effects of dietary polyphenols on gene expression in human vascular endothelial cells.Proc Nutr Soc. 2008 Feb;67(1):42-7. doi: 10.1017/S0029665108006009. Proc Nutr Soc. 2008. PMID: 18234130 Review.
Cited by
-
Prognostic influence of cyclooxygenase-2 protein and mRNA expression in node-negative breast cancer patients.BMC Cancer. 2014 Dec 15;14:952. doi: 10.1186/1471-2407-14-952. BMC Cancer. 2014. PMID: 25511800 Free PMC article.
-
Vascular Aging in Women: is Estrogen the Fountain of Youth?Front Physiol. 2012 Jun 6;3:165. doi: 10.3389/fphys.2012.00165. eCollection 2012. Front Physiol. 2012. PMID: 22685434 Free PMC article.
-
17β-estradiol inhibits Notch1 activation in murine macrophage cell line RAW 264.7.Mol Biol Rep. 2024 Nov 8;51(1):1134. doi: 10.1007/s11033-024-10058-x. Mol Biol Rep. 2024. PMID: 39514048
-
Gender-dependent correlations of carotid intima-media thickness with gene expression in blood.Transl Stroke Res. 2011 Jun;2(2):171-8. doi: 10.1007/s12975-011-0066-4. Transl Stroke Res. 2011. PMID: 22287995 Free PMC article.
-
Inhibition of Dimethylarginine Dimethylaminohydrolase (DDAH) Enzymes as an Emerging Therapeutic Strategy to Target Angiogenesis and Vasculogenic Mimicry in Cancer.Front Oncol. 2020 Jan 9;9:1455. doi: 10.3389/fonc.2019.01455. eCollection 2019. Front Oncol. 2020. PMID: 31993367 Free PMC article. Review.
References
-
- Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. - PubMed
-
- Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Pub Health. 1998;19:55–72. - PubMed
-
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. - PubMed
-
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49–57. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

