A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy
- PMID: 20011586
- PMCID: PMC2787245
- DOI: 10.1371/journal.pone.0008060
A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy
Abstract
Background: Herpes simplex virus type-2 (HSV-2) infection enhances the transmission and acquisition of human immunodeficiency virus (HIV). This occurs in symptomatic and asymptomatic stages of HSV-2 infection, suggesting that obvious herpetic lesions are not required to increase HIV spread. An animal model to investigate the underlying causes of the synergistic action of the two viruses and where preventative strategies can be tested under such complex physiological conditions is currently unavailable.
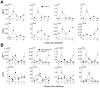
Methodology/principal findings: We set out to establish a rhesus macaque model in which HSV-2 infection increases the susceptibility to vaginal infection with a model immunodeficiency virus (simian-human immunodeficiency virus, SHIV-RT), and to more stringently test promising microbicides. HSV-2 exposure significantly increased the frequency of vaginal SHIV-RT infection (n = 6). Although cervical lesions were detected in only approximately 10% of the animals, long term HSV-2 DNA shedding was detected (in 50% of animals followed for 2 years). Vaginal HSV-2 exposure elicited local cytokine/chemokine (n = 12) and systemic low-level HSV-2-specific adaptive responses in all animals (n = 8), involving CD4(+) and CD8(+) HSV-specific T cells (n = 5). Local cytokine/chemokine responses were lower in co-infected animals, while simian immunodeficiency virus (SIV)-specific adaptive responses were comparable in naïve and HSV-2-infected animals (n = 6). Despite the increased frequency of SHIV-RT infection, a new generation microbicide gel, comprised of Carraguard(R) and a non-nucleoside reverse transcriptase inhibitor MIV-150 (PC-817), blocked vaginal SHIV-RT infection in HSV-2-exposed animals (n = 8), just as in naïve animals.
Conclusions/significance: We established a unique HSV-2 macaque model that will likely facilitate research to define how HSV-2 increases HIV transmission, and enable more rigorous evaluation of candidate anti-viral approaches in vivo.
Conflict of interest statement
Figures
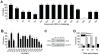
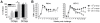
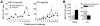

Similar articles
-
A combination microbicide gel protects macaques against vaginal simian human immunodeficiency virus-reverse transcriptase infection, but only partially reduces herpes simplex virus-2 infection after a single high-dose cochallenge.AIDS Res Hum Retroviruses. 2014 Feb;30(2):174-83. doi: 10.1089/aid.2013.0165. Epub 2013 Nov 22. AIDS Res Hum Retroviruses. 2014. PMID: 24117013 Free PMC article.
-
MZC Gel Inhibits SHIV-RT and HSV-2 in Macaque Vaginal Mucosa and SHIV-RT in Rectal Mucosa.J Acquir Immune Defic Syndr. 2017 Mar 1;74(3):e67-e74. doi: 10.1097/QAI.0000000000001167. J Acquir Immune Defic Syndr. 2017. PMID: 27552154 Free PMC article.
-
A potent combination microbicide that targets SHIV-RT, HSV-2 and HPV.PLoS One. 2014 Apr 16;9(4):e94547. doi: 10.1371/journal.pone.0094547. eCollection 2014. PLoS One. 2014. PMID: 24740100 Free PMC article.
-
Mechanistic Studies of Viral Entry: An Overview of Dendrimer-Based Microbicides As Entry Inhibitors Against Both HIV and HSV-2 Overlapped Infections.Med Res Rev. 2017 Jan;37(1):149-179. doi: 10.1002/med.21405. Epub 2016 Aug 12. Med Res Rev. 2017. PMID: 27518199 Review.
-
The Role of Tissue Resident Memory CD4 T Cells in Herpes Simplex Viral and HIV Infection.Viruses. 2021 Feb 25;13(3):359. doi: 10.3390/v13030359. Viruses. 2021. PMID: 33668777 Free PMC article. Review.
Cited by
-
Current status and prospects for development of an HSV vaccine.Vaccine. 2014 Mar 20;32(14):1553-60. doi: 10.1016/j.vaccine.2013.08.066. Epub 2013 Sep 6. Vaccine. 2014. PMID: 24016811 Free PMC article.
-
A model of genital herpes simplex virus Type 1 infection in Rhesus Macaques.J Med Primatol. 2017 Aug;46(4):121-128. doi: 10.1111/jmp.12293. J Med Primatol. 2017. PMID: 28748667 Free PMC article.
-
The nonnucleoside reverse transcriptase inhibitor MIV-150 in carrageenan gel prevents rectal transmission of simian/human immunodeficiency virus infection in macaques.J Virol. 2011 Jun;85(11):5504-12. doi: 10.1128/JVI.02422-10. Epub 2011 Mar 16. J Virol. 2011. PMID: 21411526 Free PMC article.
-
Experimental Oral Herpes Simplex Virus-1 (HSV-1) Co-infection in Simian Immunodeficiency Virus (SIV)-Infected Rhesus Macaques.Front Microbiol. 2017 Dec 5;8:2342. doi: 10.3389/fmicb.2017.02342. eCollection 2017. Front Microbiol. 2017. PMID: 29259582 Free PMC article.
-
In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition.Antiviral Res. 2014 Aug;108:88-93. doi: 10.1016/j.antiviral.2014.05.018. Epub 2014 Jun 5. Antiviral Res. 2014. PMID: 24909570 Free PMC article.
References
-
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. Jama. 2006;296:964–973. - PubMed
-
- Corey L. Herpes simplex virus type 2 and HIV-1: the dialogue between the 2 organisms continues. J Infect Dis. 2007;195:1242–1244. - PubMed
-
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006;20:73–83. - PubMed
-
- Kapiga SH, Sam NE, Bang H, Ni Q, Ao TT, et al. The role of herpes simplex virus type 2 and other genital infections in the acquisition of HIV-1 among high-risk women in northern Tanzania. J Infect Dis. 2007;195:1260–1269. - PubMed
-
- Reynolds SJ, Risbud AR, Shepherd ME, Zenilman JM, Brookmeyer RS, et al. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J Infect Dis. 2003;187:1513–1521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

