The danger signal adenosine induces persistence of chlamydial infection through stimulation of A2b receptors
- PMID: 20011598
- PMCID: PMC2788228
- DOI: 10.1371/journal.pone.0008299
The danger signal adenosine induces persistence of chlamydial infection through stimulation of A2b receptors
Abstract
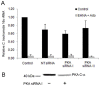
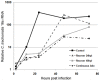
Infections with intracellular bacteria such as chlamydiae affect the majority of the world population. Infected tissue inflammation and granuloma formation help contain the short-term expansion of the invading pathogen, leading also to local tissue damage and hypoxia. However, the effects of key aspects of damaged inflamed tissues and hypoxia on continued infection with intracellular bacteria remain unknown. We find that development of Chlamydia trachomatis is reversibly retarded by prolonged exposure of infected cells to extracellular adenosine, a hallmark of hypoxia and advanced inflammation. In epithelial cells, this effect was mediated by the A2b adenosine receptor, unique in the adenosine receptor family for having a hypoxia-inducible factor (HIF1-alpha) binding site at its promoter region, and was dependent on an increase in the intracellular cAMP levels, but was independent of cAMP-dependent protein kinase (PKA). Further study of adenosine receptor signaling during intracellular bacterial infection could lead to breakthroughs in our understanding of persistent infections with these ubiquitous pathogens.
Conflict of interest statement
Figures
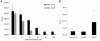


Similar articles
-
Damage/Danger Associated Molecular Patterns (DAMPs) Modulate Chlamydia pecorum and C. trachomatis Serovar E Inclusion Development In Vitro.PLoS One. 2015 Aug 6;10(8):e0134943. doi: 10.1371/journal.pone.0134943. eCollection 2015. PLoS One. 2015. PMID: 26248286 Free PMC article.
-
Cyclic AMP inhibits developmental regulation of Chlamydia trachomatis.J Bacteriol. 1986 Nov;168(2):722-7. doi: 10.1128/jb.168.2.722-727.1986. J Bacteriol. 1986. PMID: 3023286 Free PMC article.
-
Stimulation of the A2B Adenosine Receptor Subtype Enhances Connexin26 Hemichannel Activity in Small Airway Epithelial Cells.Cell Physiol Biochem. 2019;53(4):606-622. doi: 10.33594/000000160. Cell Physiol Biochem. 2019. PMID: 31550088
-
Regulation of lymphocyte function by adenosine.Arterioscler Thromb Vasc Biol. 2012 Sep;32(9):2097-103. doi: 10.1161/ATVBAHA.111.226837. Epub 2012 Jul 5. Arterioscler Thromb Vasc Biol. 2012. PMID: 22772752 Free PMC article. Review.
-
Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation.Expert Opin Ther Targets. 2009 Nov;13(11):1267-77. doi: 10.1517/14728220903241666. Expert Opin Ther Targets. 2009. PMID: 19769545 Free PMC article. Review.
Cited by
-
Chlamydia Uses K+ Electrical Signalling to Orchestrate Host Sensing, Inter-Bacterial Communication and Differentiation.Microorganisms. 2021 Jan 15;9(1):173. doi: 10.3390/microorganisms9010173. Microorganisms. 2021. PMID: 33467438 Free PMC article.
-
Reversible inhibition of Chlamydia trachomatis infection in epithelial cells due to stimulation of P2X(4) receptors.Infect Immun. 2012 Dec;80(12):4232-8. doi: 10.1128/IAI.00441-12. Epub 2012 Sep 17. Infect Immun. 2012. PMID: 22988022 Free PMC article.
-
A Cellular Assay for the Identification and Characterization of Connexin Gap Junction Modulators.Int J Mol Sci. 2021 Jan 31;22(3):1417. doi: 10.3390/ijms22031417. Int J Mol Sci. 2021. PMID: 33572565 Free PMC article.
-
Danger signal adenosine via adenosine 2a receptor stimulates growth of Porphyromonas gingivalis in primary gingival epithelial cells.Mol Oral Microbiol. 2014 Apr;29(2):67-78. doi: 10.1111/omi.12045. Epub 2014 Feb 12. Mol Oral Microbiol. 2014. PMID: 24517244 Free PMC article.
-
Blood-Brain Barrier in a Haemophilus influenzae Type a In Vitro Infection: Role of Adenosine Receptors A2A and A2B.Mol Neurobiol. 2018 Jun;55(6):5321-5336. doi: 10.1007/s12035-017-0769-y. Epub 2017 Sep 18. Mol Neurobiol. 2018. PMID: 28921456
References
-
- Belland R, Ojcius DM, Byrne GI. Chlamydia. Nature Rev Microbiol. 2004;2:530–531. - PubMed
-
- Wyrick PB. Intracellular survival by Chlamydia. Cell Microbiol. 2000;2:275–282. - PubMed
-
- Bavoil PM, Hsia R-c, Ojcius DM. Closing in on Chlamydia and its intracellular bag of tricks. Microbiol. 2000;146:2723–2731. - PubMed
-
- Roan NR, Starnbach MN. Immune-mediated control of Chlamydia infection. Cell Microbiol. 2008;10:9–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

