Angiotensin I-converting enzyme mutation (Trp1197Stop) causes a dramatic increase in blood ACE
- PMID: 20011602
- PMCID: PMC2788243
- DOI: 10.1371/journal.pone.0008282
Angiotensin I-converting enzyme mutation (Trp1197Stop) causes a dramatic increase in blood ACE
Abstract
Background: Angiotensin-converting enzyme (ACE) metabolizes many peptides and plays a key role in blood pressure regulation and vascular remodeling. Elevated ACE levels may be associated with an increased risk for different cardiovascular or respiratory diseases, including asthma. Previously, a molecular mechanism underlying a 5-fold familial increase of blood ACE was discovered: Pro1199Leu substitution enhanced the cleavage-secretion process. Carriers of this mutation were Caucasians from Europe (mostly Dutch) or had European roots.
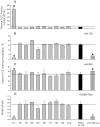
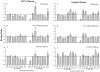
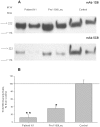
Methodology/principal findings: We have found a family of African-American descent whose affected members' blood ACE level was increased 13-fold over normal. In affected family members, codon TGG coding for Trp1197 was substituted in one allele by TGA (stop codon). As a result, half of ACE expressed in these individuals had a length of 1196 amino acids and lacked a transmembrane anchor. This ACE mutant is not trafficked to the cell membrane and is directly secreted out of cells; this mechanism apparently accounts for the high serum ACE level seen in affected individuals. A haplotype of the mutant ACE allele was determined based on 12 polymorphisms, which may help to identify other carriers of this mutation. Some but not all carriers of this mutation demonstrated airflow obstruction, and some but not all have hypertension.
Conclusions/significance: We have identified a novel Trp1197Stop mutation that results in dramatic elevation of serum ACE. Since blood ACE elevation is often taken as a marker of disease activity (sarcoidosis and Gaucher diseases), it is important for clinicians and medical scientists to be aware of alternative genetic causes of elevated blood ACE that are not apparently linked to disease.
Conflict of interest statement
Figures





References
-
- Ehlers MR, Riordan JF. Angiotensin-converting enzyme: new concepts concerning its biological role. Biochemistry. 1989;8:5311–5318. - PubMed
-
- Corvol P, Eyries M, Soubrier F. Peptidyl-dipeptidase A/Angiotensin I-converting enzyme. In: Barret A, Rawlings N, Woessner J, editors. Handbook of Proteolytic Enzymes. New York: Elsevier Academic Press; 2004. pp. 332–349.
-
- Bernstein KE, Xiao HD, Frenzel K, Li P, Shen XZ, et al. Six truisms concerning ACE and the renin-angiotensin system educed from the genetic analysis of mice. Circ Res. 2005;96:1135–1144. - PubMed
-
- Caldwell PR, Seegal BC, Hsu KC, Das H, Soffer RL. Angiotensin-converting enztme: vascular endothelial localization. Science. 1976;191:1050–1051. - PubMed
-
- Defendini R, Zimmerman EA, Weare JA, Alhenc-Gelas F, Erdos EG. Angiotensin-converting enzyme in epithelial and neuroepithelial cells. Neuroendocrinology. 1983;37:32–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

