Dielectrophoretic Separation of Cancer Cells from Blood
- PMID: 20011619
- PMCID: PMC2790288
- DOI: 10.1109/28.585856
Dielectrophoretic Separation of Cancer Cells from Blood
Abstract
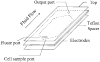
Recent measurements have demonstrated that the dielectric properties of cells depend on their type and physiological status. For example, MDA-231 human breast cancer cells were found to have a mean plasma membrane specific capacitance of 26 mF/m(2), more than double the value (11 mF/m(2)) observed for resting T-lymphocytes. When an inhomogeneous ac electric field is applied to a particle, a dielectrophoretic (DEP) force arises that depends on the particle dielectric properties. Therefore, cells having different dielectric characteristics will experience differential DEP forces when subjected to such a field. In this article, we demonstrate the use of differential DEP forces for the separation of several different cancerous cell types from blood in a dielectric affinity column. These separations were accomplished using thin, flat chambers having microelectrode arrays on the bottom wall. DEP forces generated by the application of ac fields to the electrodes were used to influence the rate of elution of cells from the chamber by hydrodynamic forces within a parabolic fluid flow profile. Electrorotation measurements were first made on the various cell types found within cell mixtures to be separated, and theoretical modeling was used to derive the cell dielectric parameters. Optimum separation conditions were then predicted from the frequency and suspension conductivity dependencies of cell DEP responses defined by these parameters. Cell separations were then undertaken for various ratios of cancerous to normal cells at different concentrations. Eluted cells were characterized in terms of separation efficiency, cell viability, and separation speed. For example, 100% efficiency was achieved for purging MDA-231 cells from blood at the tumor to normal cell ratio 1:1 x 10(5) or 1:3 x 10(5), cell viability was not compromised, and separation rates were at least 10(3) cells/s. Theoretical and experimental criteria for the design and operation of such separators are presented.
Figures


Similar articles
-
Separation of polystyrene microbeads using dielectrophoretic/gravitational field-flow-fractionation.Biophys J. 1998 May;74(5):2689-701. doi: 10.1016/S0006-3495(98)77975-5. Biophys J. 1998. PMID: 9591693 Free PMC article.
-
Cell separation on microfabricated electrodes using dielectrophoretic/gravitational field-flow fractionation.Anal Chem. 1999 Mar 1;71(5):911-8. doi: 10.1021/ac981250p. Anal Chem. 1999. PMID: 10079757
-
Cell separation by dielectrophoretic field-flow-fractionation.Anal Chem. 2000 Feb 15;72(4):832-9. doi: 10.1021/ac990922o. Anal Chem. 2000. PMID: 10701270 Free PMC article.
-
Electric field-induced effects on neuronal cell biology accompanying dielectrophoretic trapping.Adv Anat Embryol Cell Biol. 2003;173:III-IX, 1-77. doi: 10.1007/978-3-642-55469-8. Adv Anat Embryol Cell Biol. 2003. PMID: 12901336 Review.
-
Dielectrophoretic separation of bioparticles in microdevices: a review.Electrophoresis. 2014 Mar;35(5):691-713. doi: 10.1002/elps.201300424. Epub 2014 Feb 4. Electrophoresis. 2014. PMID: 24338825 Review.
Cited by
-
Benchtop technologies for circulating tumor cells separation based on biophysical properties.Biomed Res Int. 2015;2015:239362. doi: 10.1155/2015/239362. Epub 2015 Apr 21. Biomed Res Int. 2015. PMID: 25977918 Free PMC article. Review.
-
An integrated micro-electro-fluidic and protein arraying system for parallel analysis of cell responses to controlled microenvironments.Integr Biol (Camb). 2010 Sep;2(9):416-23. doi: 10.1039/c0ib00017e. Epub 2010 Aug 10. Integr Biol (Camb). 2010. PMID: 20714638 Free PMC article.
-
Rapid assessment of early biophysical changes in K562 cells during apoptosis determined using dielectrophoresis.Int J Nanomedicine. 2006;1(3):333-7. Int J Nanomedicine. 2006. PMID: 17717973 Free PMC article.
-
Sorting Lithium-Ion Battery Electrode Materials Using Dielectrophoresis.ACS Omega. 2023 Jul 14;8(29):26635-26643. doi: 10.1021/acsomega.3c04057. eCollection 2023 Jul 25. ACS Omega. 2023. PMID: 37521612 Free PMC article.
-
A Prominent Cell Manipulation Technique in BioMEMS: Dielectrophoresis.Micromachines (Basel). 2020 Nov 3;11(11):990. doi: 10.3390/mi11110990. Micromachines (Basel). 2020. PMID: 33153069 Free PMC article. Review.
References
-
- Fischer A. The use of monoclonal antibodies in allogeneic bone marrow transplantation. Br J Haematol. 1993;83:531–534. - PubMed
-
- Stout RD. Macrophage activation by T cells: Cognate and noncognate signals. Curr Opin Immunol. 1993;5(3):398–403. - PubMed
-
- Cantrell DA, Graves JD, Izquierdo M, Lucas S, Downward J. T lymphocyte activiation signals. Proc Ciba Found Symp. 1992;164:208–222. - PubMed
-
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4:269–274. - PubMed
-
- Smeland EB, Funderud S, Blomhoff HK, Egeland T. Isolation and characterization of human hematopoietic progenitor cells: An effective method for the positive selection of CD34(+) cells. Leukemia. 1992;6:845–852. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
