Calculating hematopoietic-mode-lethality risk avoidance associated with radionuclide decorporation countermeasures related to a radiological terrorism incident
- PMID: 20011652
- PMCID: PMC2790316
- DOI: 10.2203/dose-response.09-022.Scott
Calculating hematopoietic-mode-lethality risk avoidance associated with radionuclide decorporation countermeasures related to a radiological terrorism incident
Abstract
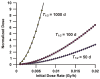
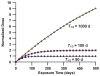
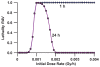
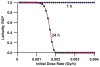
This paper provides theoretical health-risk-assessment tools that are designed to facilitate planning for and managing radiological terrorism incidents that involve ingestion exposure to bone-seeking radionuclides (e.g., radiostrontium nuclides). The focus is on evaluating lethality risk avoidance (RAV; i.e., the decrease in risk) that is associated with radionuclide decorporation countermeasures employed to remove ingested bone-seeking beta and/or gamma-emitting radionuclides from the body. To illustrate the application of tools presented, hypothetical radiostrontium decorporation scenarios were considered that involved evaluating the hematopoietic-mode-lethality RAV. For evaluating the efficacy of specific decorporation countermeasures, the lethality risk avoidance proportion (RAP; which is the RAV divided by the total lethality risk in the absence of protective countermeasures) is introduced. The lethality RAP is expected to be a useful tool for designing optimal radionuclide decorporation schemes and for identifying green, yellow and red dose-rate zones. For the green zone, essentially all of the lethality risk is expected to be avoided (RAP = 1) as a consequence of the radionuclide decorporation scheme used. For the yellow zone, some but not all of the lethality risk is expected to be avoided. For the red zone, none of the lethality risk (which equals 1) is expected to be avoided.
Keywords: Risk; countermeasures; lethality; radiation; radionuclide.
Figures

References
-
- Ainsworth EJ, Leong GF, Kendal K, Alpen EL, Albright ML. Biological Effects of Neutron and Proton Irradiation. II. IAEA; Vienna: 1964. Pulsed irradiation studies in mice, rats and dogs; pp. 15–30.
-
- Ansari A. Dirty bomb pills, shots, weeds, and spells. Health Physics News XXXII(11) 2004:1–7.
-
- ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profile for Strontium. U.S. Department of Health and Humans Services, Agency for Toxic Substances and Disease Registry. 2004 2004 Apr;
-
- Augustine AD, Godré-Lewis T, McBride W, Miller L, Pellmar RC, Rockwell S. Animal models for radiation injury, protection and therapy. Radiat Res. 2005;164:100–109. - PubMed
-
- Bateman JL, Bond VP, Robertson JS. Dose-rate dependence of early radiation effects in small mammals. Radiology. 1962;79:1008–1014. - PubMed
LinkOut - more resources
Full Text Sources

