Computation of egomotion in the macaque cerebellar vermis
- PMID: 20012388
- PMCID: PMC3640361
- DOI: 10.1007/s12311-009-0147-z
Computation of egomotion in the macaque cerebellar vermis
Abstract
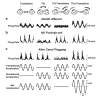
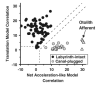
The nodulus and uvula (lobules X and IX of the vermis) receive mossy fibers from both vestibular afferents and vestibular nuclei neurons and are thought to play a role in spatial orientation. Their properties relate to a sensory ambiguity of the vestibular periphery: otolith afferents respond identically to translational (inertial) accelerations and changes in orientation relative to gravity. Based on theoretical and behavioral evidence, this sensory ambiguity is resolved using rotational cues from the semicircular canals. Recordings from the cerebellar cortex have identified a neural correlate of the brain's ability to resolve this ambiguity in the simple spike activities of nodulus/uvula Purkinje cells. This computation, which likely involves the cerebellar circuitry and its reciprocal connections with the vestibular nuclei, results from a remarkable convergence of spatially- and temporally-aligned otolith-driven and semicircular canal-driven signals. Such convergence requires a spatio-temporal transformation of head-centered canal-driven signals into an estimate of head reorientation relative to gravity. This signal must then be subtracted from the otolith-driven estimate of net acceleration to compute inertial motion. At present, Purkinje cells in the nodulus/uvula appear to encode the output of this computation. However, how the required spatio-temporal matching takes place within the cerebellar circuitry and what role complex spikes play in spatial orientation and disorientation remains unknown. In addition, the role of visual cues in driving and/or modifying simple and complex spike activity, a process potentially critical for long-term adaptation, constitutes another important direction for future studies.
Figures



References
-
- Blazquez PM, Hirata Y, Highstein SM. The vestibulo-ocular reflex as a model system for motor learning: what is the role of the cerebellum? Cerebellum. 2004;3(3):188–192. - PubMed
-
- Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci. 2004;27:581–609. - PubMed
-
- du Lac S, Raymond JL, Sejnowski TJ, Lisberger SG. Learning and memory in the vestibulo-ocular reflex. Annu Rev Neurosci. 1995;18:409–441. - PubMed
-
- Raymond JL, Lisberger SG, Mauk MD. The cerebellum: a neuronal learning machine? Science. 1996;272:1126–1131. - PubMed
-
- Barmack NH, Baughman RW, Errico P, Shojaku H. Vestibular primary afferent projection to the cerebellum of the rabbit. J Comp Neurol. 1993;327:521–534. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

