Prevalence and isotypic complexity of the anti-Chinese hamster ovary host cell protein antibodies in normal human serum
- PMID: 20013082
- PMCID: PMC2811637
- DOI: 10.1208/s12248-009-9165-5
Prevalence and isotypic complexity of the anti-Chinese hamster ovary host cell protein antibodies in normal human serum
Abstract
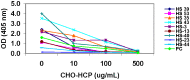
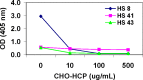
Host cell-derived protein impurities may be present at low levels in biopharmaceutical products. Antibodies to host cell proteins are present in individuals with no known exposure to these products. In this study, antibodies to drug process-specific Chinese hamster ovary host cell-derived proteins (CHO-HCP) were measured in unexposed individuals using a validated enzyme-linked immunosorbent assay. Samples that tested positive for anti-CHO-HCP reactivity were further characterized to determine the isotypes and IgG subclasses expressed in each positive individual. The specificity of the detected anti-CHO-HCP antibody isotypes was confirmed by the competitive inhibition assay and the uncoated plate specificity testing. These antibody characterization experiments revealed that the prevalent anti-CHO-HCP antibody subclasses were predominantly IgG1 (present in 66.6% of individuals) and IgG2 (60%), with 33.3% positive for IgG3 while IgG4 was detected in low abundance. Forty percent (40%) of the individuals with IgG type reactivity were also identified as IgM-positive. Anti-CHO-HCP-specific IgE was not detected in the assays used in this study. Some individuals exhibited single isotype anti-CHO-HCP reactivity; others contained a combination of multiple antibody isotypes. Data presented in this study demonstrate the isotypic complexity and the high prevalence of anti-CHO-HCP antibodies in human serum with no known exposure to CHO cell-derived therapeutic biologics.
Figures



Similar articles
-
Identification of a host cell protein impurity in therapeutic protein, P1.J Pharm Biomed Anal. 2017 Jul 15;141:32-38. doi: 10.1016/j.jpba.2017.03.065. Epub 2017 Apr 9. J Pharm Biomed Anal. 2017. PMID: 28419935
-
Isotypic analysis of grass pollen-specific immunoglobulins in human plasma. 1. Specialization of certain classes and subclasses in the immune response.Int Arch Allergy Immunol. 1993;100(1):68-78. doi: 10.1159/000236390. Int Arch Allergy Immunol. 1993. PMID: 8428167
-
Anti-Beta 2-glycoprotein I antibody isotype and IgG subclass in antiphospholipid syndrome patients.Autoimmunity. 1999 Oct;31(2):109-16. doi: 10.3109/08916939908994054. Autoimmunity. 1999. PMID: 10680749
-
Quantitation of host cell proteins in biopharmaceuticals from chinese hamster ovarian and vero cell lines using capillary electrophoresis western blots.J Pharm Biomed Anal. 2023 Sep 5;233:115420. doi: 10.1016/j.jpba.2023.115420. Epub 2023 May 3. J Pharm Biomed Anal. 2023. PMID: 37207489 Review.
-
Applications of proteomic methods for CHO host cell protein characterization in biopharmaceutical manufacturing.Curr Opin Biotechnol. 2018 Oct;53:144-150. doi: 10.1016/j.copbio.2018.01.004. Epub 2018 Feb 3. Curr Opin Biotechnol. 2018. PMID: 29414072 Review.
Cited by
-
The sodium iodide symporter is unlikely to be a thyroid/breast shared antigen.J Endocrinol Invest. 2016 Mar;39(3):323-31. doi: 10.1007/s40618-015-0368-6. Epub 2015 Aug 8. J Endocrinol Invest. 2016. PMID: 26253711
-
The future of host cell protein (HCP) identification during process development and manufacturing linked to a risk-based management for their control.Biotechnol Bioeng. 2015 Sep;112(9):1727-37. doi: 10.1002/bit.25628. Epub 2015 Jul 14. Biotechnol Bioeng. 2015. PMID: 25998019 Free PMC article. Review.
-
Specific Immune Response to Phospholipase B-Like 2 Protein, a Host Cell Impurity in Lebrikizumab Clinical Material.AAPS J. 2017 Jan;19(1):254-263. doi: 10.1208/s12248-016-9998-7. Epub 2016 Oct 13. AAPS J. 2017. PMID: 27739010
-
Immunogenicity of glycans on biotherapeutic drugs produced in plant expression systems-The taliglucerase alfa story.PLoS One. 2017 Oct 31;12(10):e0186211. doi: 10.1371/journal.pone.0186211. eCollection 2017. PLoS One. 2017. PMID: 29088235 Free PMC article. Clinical Trial.
-
Pre-existing Antibody: Biotherapeutic Modality-Based Review.AAPS J. 2016 Mar;18(2):311-20. doi: 10.1208/s12248-016-9878-1. Epub 2016 Jan 28. AAPS J. 2016. PMID: 26821802 Free PMC article. Review.
References
-
- Werner RG, Noe W, Kopp K, Schluter M. Appropriate mammalian expression systems for biopharmaceuticals. Arzneimittelforschung. 1998;48(8):870–80. - PubMed
-
- Jayapal KP, Wlaschin KF, Hu W. Recombinant protein therapeutics from CHO cells—20 years and counting. CHO Consortium SBE special section. 2007;40–7.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

