Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention
- PMID: 20013083
- PMCID: PMC2811646
- DOI: 10.1208/s12248-009-9162-8
Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention
Abstract
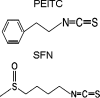
Development of cancer is a long-term and multistep process which comprises initiation, progression, and promotion stages of carcinogenesis. Conceivably, it can be targeted and interrupted along these different stages. In this context, many naturally occurring dietary compounds from our daily consumption of fruits and vegetables have been shown to possess cancer preventive effects. Phenethyl isothiocyanate (PEITC) and sulforaphane (SFN) are two of the most widely investigated isothiocyanates from the crucifers. Both have been found to be very potent chemopreventive agents in numerous animal carcinogenesis models as well as cell culture models. They exert their chemopreventive effects through regulation of diverse molecular mechanisms. In this review, we will discuss the molecular targets of PEITC and SFN potentially involved in cancer chemoprevention. These include the regulation of drug-metabolizing enzymes phase I cytochrome P450s and phase II metabolizing enzymes. In addition, the signaling pathways including Nrf2-Keap 1, anti-inflammatory NFkappaB, apoptosis, and cell cycle arrest as well as some receptors will also be discussed. Furthermore, we will also discuss the similarities and their potential differences in the regulation of these molecular targets by PEITC and SFN.
Figures

Similar articles
-
Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers.Molecules. 2018 Nov 15;23(11):2983. doi: 10.3390/molecules23112983. Molecules. 2018. PMID: 30445746 Free PMC article. Review.
-
Advances in molecular signaling mechanisms of β-phenethyl isothiocyanate antitumor effects.J Agric Food Chem. 2015 Apr 8;63(13):3311-22. doi: 10.1021/jf504627e. Epub 2015 Mar 30. J Agric Food Chem. 2015. PMID: 25798652 Review.
-
Chemopreventive activity of sulforaphane.Drug Des Devel Ther. 2018 Sep 11;12:2905-2913. doi: 10.2147/DDDT.S100534. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30254420 Free PMC article. Review.
-
Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells.Oncogene. 2005 Jun 30;24(28):4486-95. doi: 10.1038/sj.onc.1208656. Oncogene. 2005. PMID: 15856023
-
Multi-targeted prevention of cancer by sulforaphane.Cancer Lett. 2008 Oct 8;269(2):291-304. doi: 10.1016/j.canlet.2008.04.018. Epub 2008 May 27. Cancer Lett. 2008. PMID: 18504070 Free PMC article. Review.
Cited by
-
Molecular Mechanisms Underlying Anti-Inflammatory Actions of 6-(Methylsulfinyl)hexyl Isothiocyanate Derived from Wasabi (Wasabia japonica).Adv Pharmacol Sci. 2012;2012:614046. doi: 10.1155/2012/614046. Epub 2012 Aug 15. Adv Pharmacol Sci. 2012. PMID: 22927840 Free PMC article.
-
Protective effects of the antioxidant sulforaphane on behavioral changes and neurotoxicity in mice after the administration of methamphetamine.Psychopharmacology (Berl). 2012 Jul;222(1):37-45. doi: 10.1007/s00213-011-2619-3. Epub 2011 Dec 27. Psychopharmacology (Berl). 2012. PMID: 22200890
-
Broccoli Sprouts Delay Prostate Cancer Formation and Decrease Prostate Cancer Severity with a Concurrent Decrease in HDAC3 Protein Expression in Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) Mice.Curr Dev Nutr. 2017 Dec 26;2(3):nzy002. doi: 10.1093/cdn/nzy002. eCollection 2018 Mar. Curr Dev Nutr. 2017. PMID: 30019025 Free PMC article.
-
Natural Small Molecules in Breast Cancer Treatment: Understandings from a Therapeutic Viewpoint.Molecules. 2022 Mar 27;27(7):2165. doi: 10.3390/molecules27072165. Molecules. 2022. PMID: 35408561 Free PMC article. Review.
-
In Silico and In Vitro Analysis of Sulforaphane Anti-Candida Activity.Antibiotics (Basel). 2022 Dec 19;11(12):1842. doi: 10.3390/antibiotics11121842. Antibiotics (Basel). 2022. PMID: 36551499 Free PMC article.
References
-
- Wattenberg LW. Inhibition of chemical carcinogen-induced pulmonary neoplasia by butylated hydroxyanisole. J Natl Cancer Inst. 1973;50:1541–1544. - PubMed
-
- Smart RC. Chemical carcinogenesis. In: Hodgson E, editor. A textbook of modern toxicology. 3. Hoboken: Wiley; 2004. pp. 240–242.
-
- Weinstein IB. Cancer prevention: recent progress and future opportunities. Cancer Res. 1991;51:5080–5085. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

