Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18F-FDG uptake during PET: A randomized controlled trial
- PMID: 20013165
- PMCID: PMC2842563
- DOI: 10.1007/s12350-009-9179-5
Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18F-FDG uptake during PET: A randomized controlled trial
Abstract
Background: Low-carbohydrate (LC) and high-fat, low-carbohydrate (HFLC) dietary preparations may enhance (18)F-FDG-PET-based imaging of small, inflamed structures near the heart by suppressing myocardial FDG signal. We compared myocardial (18)F-FDG uptake in patients randomized to LC, HFLC, and unrestricted (UR) preparations prior to (18)F-FDG-PET.
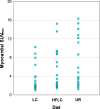
Methods and results: We randomized 63 outpatients referred for oncologic (18)F-FDG-PET to LC, HFLC, or UR dietary preparations (1:1:1 allocation) starting the evening before PET. After eating dinner according to instructions, UR and LC patients fasted until FDG injection (mean time 745 minutes for UR, 899 minutes for LC), and HFLC patients drank a fatty drink 60-70 minutes prior to FDG injection. Attenuation-corrected PET imaging was performed 60 minutes after FDG administration. Maximal myocardial standard uptake values (MyoSUV(max)) were systematically measured in axial view and compared between the three groups. Using UR patients as reference, mean MyoSUV(max) was lower in LC patients (3.3 +/- 2.7 vs 6.2 +/- 5.2, P = .03) but not in HFLC patients (5.5 +/- 4.2, P = .63). Ratios of MyoSUV(max) to liver SUV(max), calculated to control for background uptake, were not significantly different amongst the groups (1.9 +/- 2.1 LC, 2.6 +/- 2.3 HFLC, 3.6 +/- 3.5 UR).
Conclusion: In this small randomized controlled trial using UR diet as reference, LC dietary preparation followed by extended fasting resulted in significant myocardial uptake suppression.
Figures

References
-
- Engel H, Steinhart H, Buck A, Berthold T, Huch Boni RA, von Schulthess GK. Whole body PET: Physiological and artifactual fluorodeoxyglucose accumulations. J Nucl Med. 1996;37:441–446. - PubMed
-
- Shreve P, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: Physiologic and benign variants. Radiographics. 1999;19:61–77. - PubMed
-
- Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. - PubMed
-
- van der Wal AC, Das PK, Bentz van de Berg D, van der Loos CM, Becker AE. Atherosclerotic lesions in humans: In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest. 1989;61:166–170. - PubMed
-
- Ogawa M, Ishino S, Mukai T, Asano D, Teramoto N, Watabe H, et al. (18)F-FDG accumulation in atherosclerotic plaques: Immunohistochemical and PET imaging study. J Nucl Med. 2004;45:1245–1250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

