Osmolytes modulate conformational exchange in solvent-exposed regions of membrane proteins
- PMID: 20014029
- PMCID: PMC2865716
- DOI: 10.1002/pro.305
Osmolytes modulate conformational exchange in solvent-exposed regions of membrane proteins
Abstract
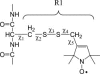
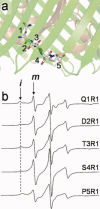
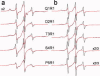
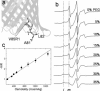
Site-directed spin labeling (SDSL) was used to investigate local structure and conformational exchange in two bacterial outer-membrane TonB-dependent transporters, BtuB and FecA. Protecting osmolytes, such as polyethylene glycols (PEGs) are known to modulate a substrate-dependent conformational equilibrium in the energy coupling motif (Ton box) of BtuB. Here, we demonstrate that a segment that is N-terminal to the Ton box in BtuB, is in conformational exchange between ordered and disordered states with or without substrate. Protecting osmolytes shift this equilibrium to favor the more ordered, folded state. However, a segment of BtuB that is C-terminal to the Ton box that is not solvent exposed is insensitive to PEGs. Protecting osmolytes also modulate a conformational equilibrium in the Ton box of FecA, with larger molecular weight PEGs producing the largest shifts in the conformational free energy. These data indicate that solvent-exposed regions of these transporters undergo conformational exchange and that regions of these transporters that are involved in protein-protein interactions sample multiple conformational substates. The sensitivity to solute provides an explanation for differences seen between two high-resolution structures of BtuB, which each likely represent one conformation from a subset of states that are normally sampled by the protein. This work also illustrates how SDSL and osmolytes may be used to characterize and quantitate conformational equilibria in membrane proteins.
Figures




Similar articles
-
Substrate-dependent transmembrane signaling in TonB-dependent transporters is not conserved.Proc Natl Acad Sci U S A. 2007 Jul 17;104(29):11975-80. doi: 10.1073/pnas.0702172104. Epub 2007 Jul 2. Proc Natl Acad Sci U S A. 2007. PMID: 17606918 Free PMC article.
-
Solutes modify a conformational transition in a membrane transport protein.Biophys J. 2006 Apr 15;90(8):2922-9. doi: 10.1529/biophysj.105.078246. Epub 2006 Jan 27. Biophys J. 2006. PMID: 16443663 Free PMC article.
-
Substrate-induced conformational changes of the periplasmic N-terminus of an outer-membrane transporter by site-directed spin labeling.Biochemistry. 2003 Feb 18;42(6):1391-400. doi: 10.1021/bi027120z. Biochemistry. 2003. PMID: 12578351
-
Identifying and quantitating conformational exchange in membrane proteins using site-directed spin labeling.Acc Chem Res. 2014 Oct 21;47(10):3102-9. doi: 10.1021/ar500228s. Epub 2014 Aug 25. Acc Chem Res. 2014. PMID: 25152957 Free PMC article. Review.
-
Energy-coupled outer membrane transport proteins and regulatory proteins.Biometals. 2007 Jun;20(3-4):219-31. doi: 10.1007/s10534-006-9072-5. Epub 2007 Mar 17. Biometals. 2007. PMID: 17370038 Review.
Cited by
-
Ligand-induced structural changes in the Escherichia coli ferric citrate transporter reveal modes for regulating protein-protein interactions.J Mol Biol. 2012 Nov 9;423(5):818-30. doi: 10.1016/j.jmb.2012.09.003. Epub 2012 Sep 11. J Mol Biol. 2012. PMID: 22982293 Free PMC article.
-
Differential backbone dynamics of companion helices in the extended helical coiled-coil domain of a bacterial chemoreceptor.Protein Sci. 2015 Nov;24(11):1764-76. doi: 10.1002/pro.2767. Epub 2015 Aug 25. Protein Sci. 2015. PMID: 26257396 Free PMC article.
-
Probing Protein Secondary Structure using EPR: Investigating a Dynamic Region of Visual Arrestin.Appl Magn Reson. 2012 Oct;43(3):405-419. doi: 10.1007/s00723-012-0369-y. Appl Magn Reson. 2012. PMID: 25419051 Free PMC article.
-
In-cell investigation of the conformational landscape of the GTPase UreG by SDSL-EPR.iScience. 2023 Sep 9;26(10):107855. doi: 10.1016/j.isci.2023.107855. eCollection 2023 Oct 20. iScience. 2023. PMID: 37766968 Free PMC article.
-
A Dynamic Protein-Protein Coupling between the TonB-Dependent Transporter FhuA and TonB.Biochemistry. 2018 Feb 13;57(6):1045-1053. doi: 10.1021/acs.biochem.7b01223. Epub 2018 Jan 26. Biochemistry. 2018. PMID: 29338257 Free PMC article.
References
-
- Frauenfelder H, Sligar SG, Wolynes PG. The energy landscapes and motions of proteins. Science. 1991;254:1598–1603. - PubMed
-
- Henzler-Wildman K, Kern D. Dynamic personalities of proteins. Nature. 2007;450:964–972. - PubMed
-
- Swain JF, Gierasch LM. The changing landscape of protein allostery. Curr Opin Struct Biol. 2006;16:102–108. - PubMed
-
- Henzler-Wildman KA, Lei M, Thai V, Kerns SJ, Karplus M, Kern D. A hierarchy of timescales in protein dynamics is linked to enzyme catalysis. Nature. 2007;450:913–916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

