The zebrafish dyrk1b gene is important for endoderm formation
- PMID: 20014342
- PMCID: PMC2806492
- DOI: 10.1002/dvg.20578
The zebrafish dyrk1b gene is important for endoderm formation
Abstract
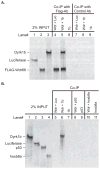
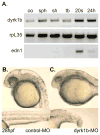
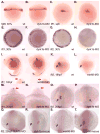
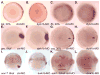
Nodal-signaling is required for specification of mesoderm, endoderm, establishing left-right asymmetry, and craniofacial development. Wdr68 is a WD40-repeat domain-containing protein recently shown to be required for endothelin-1 (edn1) expression and subsequent lower jaw development. Previous reports detected the Wdr68 protein in multiprotein complexes containing mammalian members of the dual-specificity tyrosine-regulated kinase (dyrk) family. Here we describe the characterization of the zebrafish dyrk1b homolog. We report the detection of a physical interaction between Dyrk1b and Wdr68. We also found perturbations of nodal signaling in dyrk1b antisense morpholino knockdown (dyrk1b-MO) animals. Specifically, we found reduced expression of lft1 and lft2 (lft1/2) during gastrulation and a near complete loss of the later asymmetric lft1/2 expression domains. Although wdr68-MO animals did not display lft1/2 expression defects during gastrulation, they displayed a near complete loss of the later asymmetric lft1/2 expression domains. While expression of ndr1 was not substantially effected during gastrulation, ndr2 expression was moderately reduced in dyrk1b-MO animals. Analysis of additional downstream components of the nodal signaling pathway in dyrk1b-MO animals revealed modestly expanded expression of the dorsal axial mesoderm marker gsc while the pan-mesodermal marker bik was largely unaffected. The endodermal markers cas and sox17 were also moderately reduced in dyrk1b-MO animals. Notably, and similar to defects previously reported for wdr68 mutant animals, we also found reduced expression of the pharyngeal pouch marker edn1 in dyrk1b-MO animals. Taken together, these data reveal a role for dyrk1b in endoderm formation and craniofacial patterning in the zebrafish.
Figures


Similar articles
-
Wdr68 Mediates Dorsal and Ventral Patterning Events for Craniofacial Development.PLoS One. 2016 Nov 23;11(11):e0166984. doi: 10.1371/journal.pone.0166984. eCollection 2016. PLoS One. 2016. PMID: 27880803 Free PMC article.
-
Zebrafish Cdx1b modulates epithalamic asymmetry by regulating ndr2 and lft1 expression.Dev Biol. 2021 Feb;470:21-36. doi: 10.1016/j.ydbio.2020.11.001. Epub 2020 Nov 13. Dev Biol. 2021. PMID: 33197427
-
Embryonic mesoderm and endoderm induction requires the actions of non-embryonic Nodal-related ligands and Mxtx2.Development. 2011 Feb;138(4):787-95. doi: 10.1242/dev.058974. Development. 2011. PMID: 21266414 Free PMC article.
-
The maternal coordinate system: Molecular-genetics of embryonic axis formation and patterning in the zebrafish.Curr Top Dev Biol. 2020;140:341-389. doi: 10.1016/bs.ctdb.2020.05.002. Epub 2020 Jun 16. Curr Top Dev Biol. 2020. PMID: 32591080 Review.
-
Zebrafish gastrulation: Putting fate in motion.Curr Top Dev Biol. 2020;136:343-375. doi: 10.1016/bs.ctdb.2019.10.009. Epub 2019 Dec 27. Curr Top Dev Biol. 2020. PMID: 31959295 Review.
Cited by
-
Genetic Analysis of Candida albicans Filamentation by the Iron Chelator BPS Reveals a Role for a Conserved Kinase-WD40 Protein Pair.J Fungi (Basel). 2024 Jan 22;10(1):83. doi: 10.3390/jof10010083. J Fungi (Basel). 2024. PMID: 38276029 Free PMC article.
-
Screen identifies DYRK1B network as mediator of transcription repression on damaged chromatin.Proc Natl Acad Sci U S A. 2020 Jul 21;117(29):17019-17030. doi: 10.1073/pnas.2002193117. Epub 2020 Jul 1. Proc Natl Acad Sci U S A. 2020. PMID: 32611815 Free PMC article.
-
DCAF7 is required for maintaining the cellular levels of ERCC1-XPF and nucleotide excision repair.Biochem Biophys Res Commun. 2019 Oct 29;519(1):204-210. doi: 10.1016/j.bbrc.2019.08.147. Epub 2019 Sep 4. Biochem Biophys Res Commun. 2019. PMID: 31493872 Free PMC article.
-
Wdr68 Mediates Dorsal and Ventral Patterning Events for Craniofacial Development.PLoS One. 2016 Nov 23;11(11):e0166984. doi: 10.1371/journal.pone.0166984. eCollection 2016. PLoS One. 2016. PMID: 27880803 Free PMC article.
-
DCAF7/WDR68 is required for normal levels of DYRK1A and DYRK1B.PLoS One. 2018 Nov 29;13(11):e0207779. doi: 10.1371/journal.pone.0207779. eCollection 2018. PLoS One. 2018. PMID: 30496304 Free PMC article.
References
-
- Alexander J, Stainier DY. A molecular pathway leading to endoderm formation in zebrafish. Curr Biol. 1999;9:1147–1157. - PubMed
-
- Chen Y, Schier AF. Lefty proteins are long-range inhibitors of squint-mediated nodal signaling. Curr Biol. 2002;12:2124–2128. - PubMed
-
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–824. - PubMed
-
- Clouthier DE, Schilling TF. Understanding endothelin-1 function during craniofacial development in the mouse and zebrafish. Birth Defects Res C Embryo Today. 2004;72:190–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

