Semi-permeable membrane retention of synovial fluid lubricants hyaluronan and proteoglycan 4 for a biomimetic bioreactor
- PMID: 20014439
- PMCID: PMC2913685
- DOI: 10.1002/bit.22645
Semi-permeable membrane retention of synovial fluid lubricants hyaluronan and proteoglycan 4 for a biomimetic bioreactor
Abstract
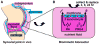
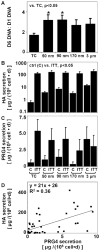
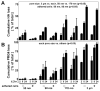
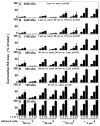
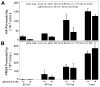
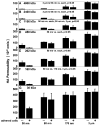
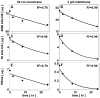
Synovial fluid (SF) contains lubricant macromolecules, hyaluronan (HA), and proteoglycan 4 (PRG4). The synovium not only contributes lubricants to SF through secretion by synoviocyte lining cells, but also concentrates lubricants in SF due to its semi-permeable nature. A membrane that recapitulates these synovium functions may be useful in a bioreactor system for generating a bioengineered fluid (BF) similar to native SF. The objectives were to analyze expanded polytetrafluoroethylene membranes with pore sizes of 50 nm, 90 nm, 170 nm, and 3 microm in terms of (1) HA and PRG4 secretion rates by adherent synoviocytes, and (2) the extent of HA and PRG4 retention with or without synoviocytes adherent on the membrane. Experiment 1: Synoviocytes were cultured on tissue culture (TC) plastic or membranes +/- IL-1beta + TGF-beta1 + TNF-alpha, a cytokine combination that stimulates lubricant synthesis. HA and PRG4 secretion rates were assessed by analysis of medium. Experiment 2: Bioreactors were fabricated to provide a BF compartment enclosed by membranes +/- adherent synoviocytes, and an external compartment of nutrient fluid (NF). A solution with HA (1 mg/mL, MW ranging from 30 to 4,000 kDa) or PRG4 (50 microg/mL) was added to the BF compartment, and HA and PRG4 loss into the NF compartment after 2, 8, and 24 h was determined. Lubricant loss kinetics were analyzed to estimate membrane permeability. Experiment 1: Cytokine-regulated HA and PRG4 secretion rates on membranes were comparable to those on TC plastic. Experiment 2: Transport of HA and PRG4 across membranes was lowest with 50 nm membranes and highest with 3 microm membranes, and transport of high MW HA was decreased by adherent synoviocytes (for 50 and 90 nm membranes). The permeability to HA mixtures for 50 nm membranes was approximately 20 x 10(-8) cm/s (- cells) and approximately 5 x 10(-8) cm/s (+ cells), for 90 nm membranes was approximately 35 x 10(-8) cm/s (- cells) and approximately 19 x 10(-8) cm/s (+ cells), for 170 nm membranes was approximately 74 x 10(-8) cm/s (+/- cells), and for 3 microm membranes was approximately 139 x 10(-8) cm/s (+/- cells). The permeability of 450 kDa HA was approximately 40x lower than that of 30 kDa HA for 50 nm membranes, but only approximately 2.5x lower for 3 microm membranes. The permeability of 4,000 kDa HA was approximately 250x lower than that of 30 kDa HA for 50 nm membranes, but only approximately 4x lower for 3 microm membranes. The permeability for PRG4 was approximately 4 x 10(-8) cm/s for 50 nm membranes, approximately 48 x 10(-8) cm/s for 90 nm membranes, approximately 144 x 10(-8) cm/s for 170 nm membranes, and approximately 336 x 10(-8) cm/s for 3 microm membranes. The associated loss across membranes after 24 h ranged from 3% to 92% for HA, and from 3% to 93% for PRG4. These results suggest that semi-permeable membranes may be used in a bioreactor system to modulate lubricant retention in a bioengineered SF, and that synoviocytes adherent on the membranes may serve as both a lubricant source and a barrier for lubricant transport.
Figures
Similar articles
-
Interactive cytokine regulation of synoviocyte lubricant secretion.Tissue Eng Part A. 2010 Apr;16(4):1329-37. doi: 10.1089/ten.TEA.2009.0210. Tissue Eng Part A. 2010. PMID: 19908966 Free PMC article.
-
A model of synovial fluid lubricant composition in normal and injured joints.Eur Cell Mater. 2007 Mar 6;13:26-39. doi: 10.22203/ecm.v013a03. Eur Cell Mater. 2007. PMID: 17340555
-
cAMP attenuates TGF-β's profibrotic responses in osteoarthritic synoviocytes: involvement of hyaluronan and PRG4.Am J Physiol Cell Physiol. 2018 Sep 1;315(3):C432-C443. doi: 10.1152/ajpcell.00041.2018. Epub 2018 Jun 13. Am J Physiol Cell Physiol. 2018. PMID: 29898378 Free PMC article.
-
Rheological effects of macromolecular interactions in synovial fluid.Biorheology. 2016 Jul 29;53(2):49-67. doi: 10.3233/BIR-15104. Biorheology. 2016. PMID: 27472842 Review.
-
Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent?Semin Arthritis Rheum. 2002 Aug;32(1):10-37. doi: 10.1053/sarh.2002.33720. Semin Arthritis Rheum. 2002. PMID: 12219318 Review.
Cited by
-
Moderate Physical Activity as a Prevention Method for Knee Osteoarthritis and the Role of Synoviocytes as Biological Key.Int J Mol Sci. 2019 Jan 25;20(3):511. doi: 10.3390/ijms20030511. Int J Mol Sci. 2019. PMID: 30691048 Free PMC article.
-
Toward understanding the role of cartilage particulates in synovial inflammation.Osteoarthritis Cartilage. 2017 Aug;25(8):1353-1361. doi: 10.1016/j.joca.2017.03.015. Epub 2017 Mar 30. Osteoarthritis Cartilage. 2017. PMID: 28365462 Free PMC article.
-
Synovium friction properties are influenced by proteoglycan content.J Biomech. 2024 Sep;174:112272. doi: 10.1016/j.jbiomech.2024.112272. Epub 2024 Aug 10. J Biomech. 2024. PMID: 39146899 Free PMC article.
-
Engineering superficial zone features in tissue engineered cartilage.Biotechnol Bioeng. 2013 May;110(5):1476-86. doi: 10.1002/bit.24799. Epub 2012 Dec 27. Biotechnol Bioeng. 2013. PMID: 23239161 Free PMC article.
-
Fibroblast-like synoviocyte mechanosensitivity to fluid shear is modulated by interleukin-1α.J Biomech. 2017 Jul 26;60:91-99. doi: 10.1016/j.jbiomech.2017.06.011. Epub 2017 Jun 28. J Biomech. 2017. PMID: 28716465 Free PMC article.
References
-
- Afify A, Lynne LC, Howell L. Correlation of cytologic examination with ELISA assays for hyaluronan and soluble CD44v6 levels in evaluation of effusions. Diagn Cytopathol. 2007;35(2):105–110. - PubMed
-
- Albelda SM, Sampson PM, Haselton FR, McNiff JM, Mueller SN, Williams SK, Fishman AP, Levine EM. Permeability characteristics of cultured endothelial cell monolayers. J Appl Physiol. 1988;64(1):308–322. - PubMed
-
- Alvaro-Gracia JM, Zvaifler NJ, Firestein GS. Cytokines in chronic inflammatory arthritis. V. Mutual antagonism between interferon-gamma and tumor necrosis factor-alpha on HLA-DR expression, proliferation, collagenase production, and granulocyte macrophage colony-stimulating factor production by rheumatoid arthritis synoviocytes. J Clin Invest. 1990;86(6):1790–1798. - PMC - PubMed
-
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. - PubMed
-
- Asari A, Miyauchi S, Matsuzaka S, Ito T, Kominami E, Uchiyama Y. Molecular weight-dependent effects of hyaluronate on the arthritic synovium. Arch Histol Cytol. 1998;61(2):125–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

