Neoadjuvant chemoradiotherapy for resectable esophageal carcinoma: a meta-analysis
- PMID: 20014464
- PMCID: PMC2795187
- DOI: 10.3748/wjg.15.5983
Neoadjuvant chemoradiotherapy for resectable esophageal carcinoma: a meta-analysis
Abstract
Aim: To compare neoadjuvant chemoradiotherapy and surgery with surgery alone for resectable esophageal carcinoma.
Methods: We used MEDLINE and EMBASE databases to identify eligible studies and manual searches were done to ensure no studies were missed. Trial validity assessment was performed and a trial quality score was assigned.
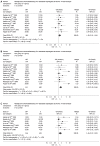
Results: Eleven randomized controlled trials (RCTs) including 1308 patients were selected. Neoadjuvant chemoradiotherapy significantly improved the overall survival compared with surgery alone. Odds ratio (OR) [95% confidence interval (CI), P value], expressed as neoadjuvant chemoradiotherapy and surgery vs surgery alone, was 1.28 (1.01-1.64, P = 0.05) for 1-year survival, 1.78 (1.20-2.66, P = 0.004) for 3-year survival, and 1.46 (1.07-1.99, P = 0.02) for 5-year survival. Postoperative mortality increased in patients treated by neoadjuvant chemoradiotherapy (OR: 1.68, 95% CI: 1.03-2.73, P = 0.04), but incidence of postoperative complications was similar in two groups (OR: 1.14, 95% CI: 0.88-1.49, P = 0.32). Neoadjuvant chemoradiotherapy lowered the local-regional cancer recurrence (OR: 0.64, 95% CI: 0.41-0.99, P = 0.04), but incidence of distant cancer recurrence was similar (OR: 0.94, 95% CI: 0.68-1.31, P = 0.73). Histological subgroup analysis indicated that esophageal squamous cell carcinoma did not benefit from neoadjuvant chemoradiotherapy, OR (95% CI, P value) was 1.16 (0.85-1.57, P = 0.34) for 1-year survival, 1.34 (0.98-1.82, P = 0.07) for 3-year survival and 1.41 (0.98-2.02, P = 0.06) for 5-year survival.
Conclusion: Neoadjuvant chemoradiotherapy can raise the survival rate of patients with esophageal adenocarcinoma.
Keywords: Esophageal carcinoma; Meta-analysis; Neoadjuvant chemoradiotherapy; Randomized controlled trial.
Figures
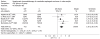



Similar articles
-
Effect of neoadjuvant chemoradiotherapy on prognosis and surgery for esophageal carcinoma.World J Gastroenterol. 2009 Oct 21;15(39):4962-8. doi: 10.3748/wjg.15.4962. World J Gastroenterol. 2009. PMID: 19842230 Free PMC article. Review.
-
Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis.Lancet Oncol. 2007 Mar;8(3):226-34. doi: 10.1016/S1470-2045(07)70039-6. Lancet Oncol. 2007. PMID: 17329193
-
A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer.Am J Surg. 2003 Jun;185(6):538-43. doi: 10.1016/s0002-9610(03)00066-7. Am J Surg. 2003. PMID: 12781882
-
A meta-analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer.Am J Surg. 2002 Mar;183(3):274-9. doi: 10.1016/s0002-9610(02)00795-x. Am J Surg. 2002. PMID: 11943125
-
[Neoadjuvant chemoradiotherapy combined with operation vs. operation alone for resectable esophageal cancer: Meta-analysis on randomized controlled trials].Zhonghua Wei Chang Wai Ke Za Zhi. 2017 Jul 25;20(7):809-815. Zhonghua Wei Chang Wai Ke Za Zhi. 2017. PMID: 28722096 Chinese.
Cited by
-
New and emerging combination therapies for esophageal cancer.Cancer Manag Res. 2013 Jun 27;5:133-46. doi: 10.2147/CMAR.S32199. Print 2013. Cancer Manag Res. 2013. PMID: 23869177 Free PMC article.
-
Support vector machine-based nomogram predicts postoperative distant metastasis for patients with oesophageal squamous cell carcinoma.Br J Cancer. 2013 Sep 3;109(5):1109-16. doi: 10.1038/bjc.2013.379. Epub 2013 Aug 13. Br J Cancer. 2013. PMID: 23942069 Free PMC article.
-
Prognostic impact of lymph node involvement and the extent of lymphadenectomy (LAD) in adenocarcinoma of the esophagogastric junction (AEG).Langenbecks Arch Surg. 2013 Oct;398(7):973-81. doi: 10.1007/s00423-013-1101-6. Epub 2013 Jul 26. Langenbecks Arch Surg. 2013. PMID: 23887283
-
Imaging strategies in the management of oesophageal cancer: what's the role of MRI?Eur Radiol. 2013 Jul;23(7):1753-65. doi: 10.1007/s00330-013-2773-6. Epub 2013 Feb 13. Eur Radiol. 2013. PMID: 23404138 Review.
-
Aggressive surgical resection does not improve survival in operable esophageal squamous cell carcinoma with N2-3 status.World J Gastroenterol. 2015 Jul 28;21(28):8644-52. doi: 10.3748/wjg.v21.i28.8644. World J Gastroenterol. 2015. PMID: 26229406 Free PMC article.
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Parkin DM, Muir CS. Cancer Incidence in Five Continents. Comparability and quality of data. IARC Sci Publ. 1992:45–173. - PubMed
-
- Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:450–464. - PubMed
-
- Khushalani N. Cancer of the esophagus and stomach. Mayo Clin Proc. 2008;83:712–722. - PubMed
-
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

