A unique modulator of endoplasmic reticulum stress-signalling pathways: the novel pharmacological properties of amiloride in glial cells
- PMID: 20015086
- PMCID: PMC2825364
- DOI: 10.1111/j.1476-5381.2009.00544.x
A unique modulator of endoplasmic reticulum stress-signalling pathways: the novel pharmacological properties of amiloride in glial cells
Abstract
Background and purpose: Stress on the endoplasmic reticulum (ER) can trigger rescuer responses such as the unfolded protein response (UPR). However, pharmacological modulators of these ER-regulated stress responses are not well understood. In the present study, we found that amiloride, a potassium-sparing diuretic, has unique properties relating to such stress.
Experimental approach: We treated mouse primary cultured glial cells with amiloride, in the absence and presence of the ER stress-inducing reagents tunicamycin (Tm) or dithiothreitol, and measured UPR and ER stress-induced cell death. IRE1alpha phosphorylation, eIF2alpha phosphorylation, X-box binding protein 1 (XBP1) splicing, glucose regulated protein 78 (GRP78) and CCAAT/enhancer-binding protein homologous protein (CHOP) expression by reverse transcription-polymerase chain reaction and Western blotting were used to assess UPR and lactate dehydrogenase activity was determined to measure ER stress-induced cell death.
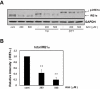
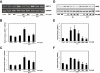
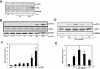
Key results: Amiloride completely inhibited ER stress-induced activation of IRE1alpha, an ER-localized stress sensor protein, splicing of XBP1, and subsequent expression of GRP78 at the mRNA and protein levels. ER stress induces the phosphorylation of eIF2alpha, leading to the expression of CHOP or an attenuation of translation in cells. Surprisingly, treatment with amiloride alone markedly promoted the phosphorylation but actually inhibited ER stress-induced CHOP expression. Finally, we found that amiloride (200 microM) synergistically enhanced ER stress-induced cell death, which was mediated through caspases. On the other hand, a low dose of amiloride (20 microM) significantly prevented Tm-induced cell death.
Conclusions and implications: These results suggest that amiloride can modulate UPR. They also suggest amiloride to be an important pharmacological agent and provide basic information for understanding and preventing ER stress-related diseases.
Figures



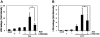
References
-
- Arias RL, Sung ML, Vasylyev D, Zhang MY, Albinson K, Kubek K, et al. Amiloride is neuroprotective in an MPTP model of Parkinson's disease. Neurobiol Dis. 2008;31:334–341. - PubMed
-
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. - PubMed
-
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, et al. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

