Isolation and pharmacological characterization of AdTx1, a natural peptide displaying specific insurmountable antagonism of the alpha1A-adrenoceptor
- PMID: 20015090
- PMCID: PMC2825353
- DOI: 10.1111/j.1476-5381.2009.00532.x
Isolation and pharmacological characterization of AdTx1, a natural peptide displaying specific insurmountable antagonism of the alpha1A-adrenoceptor
Abstract
Background and purpose: Venoms are a rich source of ligands for ion channels, but very little is known about their capacity to modulate G-protein coupled receptor (GPCR) activity. We developed a strategy to identify novel toxins targeting GPCRs.
Experimental approach: We studied the interactions of mamba venom fractions with alpha(1)-adrenoceptors in binding experiments with (3)H-prazosin. The active peptide (AdTx1) was sequenced by Edman degradation and mass spectrometry fragmentation. Its synthetic homologue was pharmacologically characterized by binding experiments using cloned receptors and by functional experiments on rabbit isolated prostatic smooth muscle.
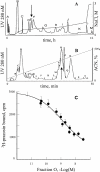
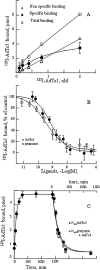
Key results: AdTx1, a 65 amino-acid peptide stabilized by four disulphide bridges, belongs to the three-finger-fold peptide family. It has subnanomolar affinity (K(i)= 0.35 nM) and high specificity for the human alpha(1A)-adrenoceptor subtype. We showed high selectivity and affinity (K(d)= 0.6 nM) of radio-labelled AdTx1 in direct binding experiments and revealed a slow association constant (k(on)= 6 x 10(6).M(-1).min(-1)) with an unusually stable alpha(1A)-adrenoceptor/AdTx1 complex (t(1/2diss)= 3.6 h). AdTx1 displayed potent insurmountable antagonism of phenylephrine's actions in vitro (rabbit isolated prostatic muscle) at concentrations of 10 to 100 nM.
Conclusions and implications: AdTx1 is the most specific and selective peptide inhibitor for the alpha(1A)-adrenoceptor identified to date. It displays insurmountable antagonism, acting as a potent relaxant of smooth muscle. Its peptidic nature can be exploited to develop new tools, as a radio-labelled-AdTx1 or a fluoro-labelled-AdTx1. Identification of AdTx1 thus offers new perspectives for developing new drugs for treating benign prostatic hyperplasia.
Figures



Similar articles
-
Orthosteric binding of ρ-Da1a, a natural peptide of snake venom interacting selectively with the α1A-adrenoceptor.PLoS One. 2013 Jul 25;8(7):e68841. doi: 10.1371/journal.pone.0068841. Print 2013. PLoS One. 2013. PMID: 23935897 Free PMC article.
-
Identification of a novel snake peptide toxin displaying high affinity and antagonist behaviour for the α2-adrenoceptors.Br J Pharmacol. 2010 Nov;161(6):1361-74. doi: 10.1111/j.1476-5381.2010.00966.x. Br J Pharmacol. 2010. PMID: 20659106 Free PMC article.
-
Molecular mechanism of human α1A-adrenoceptor inhibition by Mamba snake toxin AdTx1.Commun Biol. 2025 Jul 16;8(1):1055. doi: 10.1038/s42003-025-08405-0. Commun Biol. 2025. PMID: 40670604 Free PMC article.
-
Preclinical pharmacology of alpha1-adrenoceptor antagonists.Eur Urol. 1999;36 Suppl 1:35-41; discussion 65. doi: 10.1159/000052316. Eur Urol. 1999. PMID: 10393471 Review.
-
Adrenoceptor pharmacology: urogenital applications.Eur Urol. 1999;36 Suppl 1:17-22. doi: 10.1159/000052313. Eur Urol. 1999. PMID: 10393468 Review.
Cited by
-
Effects of ρ-Da1a a peptidic α(1) (A) -adrenoceptor antagonist in human isolated prostatic adenoma and anaesthetized rats.Br J Pharmacol. 2013 Feb;168(3):618-31. doi: 10.1111/j.1476-5381.2012.02231.x. Br J Pharmacol. 2013. PMID: 23005263 Free PMC article.
-
G-Protein Coupled Receptors Targeted by Analgesic Venom Peptides.Toxins (Basel). 2017 Nov 16;9(11):372. doi: 10.3390/toxins9110372. Toxins (Basel). 2017. PMID: 29144441 Free PMC article. Review.
-
Orthosteric binding of ρ-Da1a, a natural peptide of snake venom interacting selectively with the α1A-adrenoceptor.PLoS One. 2013 Jul 25;8(7):e68841. doi: 10.1371/journal.pone.0068841. Print 2013. PLoS One. 2013. PMID: 23935897 Free PMC article.
-
Isolation, functional characterization and proteomic identification of CC2-PLA₂ from Cerastes cerastes venom: a basic platelet-aggregation-inhibiting factor.Protein J. 2014 Feb;33(1):61-74. doi: 10.1007/s10930-013-9534-x. Protein J. 2014. PMID: 24384926
-
Engineering of three-finger fold toxins creates ligands with original pharmacological profiles for muscarinic and adrenergic receptors.PLoS One. 2012;7(6):e39166. doi: 10.1371/journal.pone.0039166. Epub 2012 Jun 14. PLoS One. 2012. PMID: 22720062 Free PMC article.
References
-
- Banerjee Y, Mizuguchi J, Iwanaga S, Kini RM. Hemextin AB complex, a unique anticoagulant protein complex from Hemachatus haemachatus (African Ringhals cobra) venom that inhibits clot initiation and factor VIIa activity. J Biol Chem. 2005;280:42601–42611. - PubMed
-
- Birdsall NJ, Lazareno S. Allosterism at muscarinic receptors: ligands and mechanisms. Mini Rev Med Chem. 2005;5:523–543. - PubMed
-
- Bradley KN. Muscarinic toxins from the green mamba. Pharmacol Ther. 2000;85:87–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

