Pharmacology of epigenetics in brain disorders
- PMID: 20015091
- PMCID: PMC2825351
- DOI: 10.1111/j.1476-5381.2009.00526.x
Pharmacology of epigenetics in brain disorders
Abstract
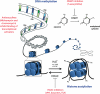
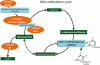
Epigenetics is a rapidly growing field and holds great promise for a range of human diseases, including brain disorders such as Rett syndrome, anxiety and depressive disorders, schizophrenia, Alzheimer disease and Huntington disease. This review is concerned with the pharmacology of epigenetics to treat disorders of the epigenome whether induced developmentally or manifested/acquired later in life. In particular, we will focus on brain disorders and their treatment by drugs that modify the epigenome. While the use of DNA methyl transferase inhibitors and histone deacetylase inhibitors in in vitro and in vivo models have demonstrated improvements in disease-related deficits, clinical trials in humans have been less promising. We will address recent advances in our understanding of the complexity of the epigenome with its many molecular players, and discuss evidence for a compromised epigenome in the context of an ageing or diseased brain. We will also draw on examples of species differences that may exist between humans and model systems, emphasizing the need for more robust pre-clinical testing. Finally, we will discuss fundamental issues to be considered in study design when targeting the epigenome.
Figures
Similar articles
-
Epigenetic-based therapies in the preclinical and clinical treatment of Huntington's disease.Int J Biochem Cell Biol. 2015 Oct;67:45-8. doi: 10.1016/j.biocel.2015.04.009. Epub 2015 Apr 30. Int J Biochem Cell Biol. 2015. PMID: 25936670 Review.
-
Epigenetic dysregulation in cognitive disorders.Eur J Neurosci. 2009 Jul;30(1):1-8. doi: 10.1111/j.1460-9568.2009.06787.x. Epub 2009 Jun 10. Eur J Neurosci. 2009. PMID: 19508697 Review.
-
Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders.Curr Opin Pharmacol. 2008 Feb;8(1):57-64. doi: 10.1016/j.coph.2007.12.002. Curr Opin Pharmacol. 2008. PMID: 18206423 Free PMC article. Review.
-
Epigenetic Mechanisms in Psychiatric Diseases and Epigenetic Therapy.Drug Dev Res. 2016 Nov;77(7):407-413. doi: 10.1002/ddr.21340. Epub 2016 Sep 4. Drug Dev Res. 2016. PMID: 27594444 Review.
-
Epigenetics and complex disease: from etiology to new therapeutics.Annu Rev Pharmacol Toxicol. 2008;48:257-76. doi: 10.1146/annurev.pharmtox.48.113006.094731. Annu Rev Pharmacol Toxicol. 2008. PMID: 17883328 Review.
Cited by
-
Epidemiology and genetics of common mental disorders in the general population: the PEGASUS-Murcia project.BMJ Open. 2013 Dec 3;3(12):e004035. doi: 10.1136/bmjopen-2013-004035. BMJ Open. 2013. PMID: 24302509 Free PMC article.
-
Understanding epigenetic architecture of suicide neurobiology: A critical perspective.Neurosci Biobehav Rev. 2017 Jan;72:10-27. doi: 10.1016/j.neubiorev.2016.10.031. Epub 2016 Nov 9. Neurosci Biobehav Rev. 2017. PMID: 27836463 Free PMC article. Review.
-
Quantitative comparison of the efficacy of various compounds in lowering intracellular cholesterol levels in Niemann-Pick type C fibroblasts.PLoS One. 2012;7(10):e48561. doi: 10.1371/journal.pone.0048561. Epub 2012 Oct 29. PLoS One. 2012. PMID: 23144769 Free PMC article.
-
The Molecular Biology of Susceptibility to Post-Traumatic Stress Disorder: Highlights of Epigenetics and Epigenomics.Int J Mol Sci. 2021 Oct 4;22(19):10743. doi: 10.3390/ijms221910743. Int J Mol Sci. 2021. PMID: 34639084 Free PMC article. Review.
-
Genetics of schizophrenia from a clinicial perspective.Int Rev Psychiatry. 2012 Oct;24(5):393-404. doi: 10.3109/09540261.2012.709178. Int Rev Psychiatry. 2012. PMID: 23057976 Free PMC article. Review.
References
-
- Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, et al. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:60–66. - PubMed
-
- Abelson JF, Kwan KY, O'Roak BJ, Baek DY, Stillman AA, Morgan TM, et al. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science. 2005;310:317–320. - PubMed
-
- Addington AM, Gornick M, Duckworth J, Sporn A, Gogtay N, Bobb A, et al. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol Psychiatry. 2005;10:581–588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

