A novel choline cotransporter sequestration compartment in cholinergic neurons revealed by selective endosomal ablation
- PMID: 20015153
- PMCID: PMC2821660
- DOI: 10.1111/j.1471-4159.2009.06543.x
A novel choline cotransporter sequestration compartment in cholinergic neurons revealed by selective endosomal ablation
Erratum in
- J Neurochem.2010 Apr;113(1):285
Abstract
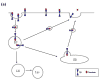
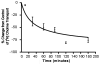
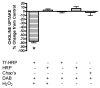
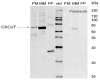
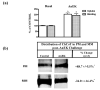
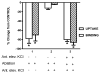
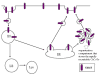
The sodium-dependent, high affinity choline transporter - choline cotransporter - (ChCoT, aka: cho-1, CHT1, CHT) undergoes constitutive and regulated trafficking between the plasma membrane and cytoplasmic compartments. The pathways and regulatory mechanisms of this trafficking are not well understood. We report herein studies involving selective endosomal ablation to further our understanding of the trafficking of the ChCoT. Selective ablation of early sorting and recycling endosomes resulted in a decrease of approximately 75% of [3H]choline uptake and approximately 70% of [3H]hemicholinium-3 binding. Western blot analysis showed that ablation produced a similar decrease in ChCoTs in the plasma membrane subcellular fraction. The time frame for this loss was approximately 2 h which has been shown to be the constitutive cycling time for ChCoTs in this tissue. Ablation appears to be dependent on the intracellular cycling of transferrin-conjugated horseradish peroxidase and the selective deposition of transferrin-conjugated horseradish peroxidase in early endosomes, both sorting and recycling. Ablated brain slices retained their capacity to recruit via regulated trafficking ChCoTs to the plasma membrane. This recruitment of ChCoTs suggests that the recruitable compartment is distinct from the early endosomes. It will be necessary to do further studies to identify the novel sequestration compartment supportive of the ChCoT regulated trafficking.
Figures


Similar articles
-
Hemicholinium-3 mustard reveals two populations of cycling choline cotransporters in Limulus.Neuroscience. 2001;102(4):969-78. doi: 10.1016/s0306-4522(00)00534-0. Neuroscience. 2001. PMID: 11182258
-
Activity and subcellular trafficking of the sodium-coupled choline transporter CHT is regulated acutely by peroxynitrite.Mol Pharmacol. 2008 Mar;73(3):801-12. doi: 10.1124/mol.107.040881. Epub 2007 Oct 30. Mol Pharmacol. 2008. PMID: 17971421
-
The hemicholinium-3 sensitive high affinity choline transporter is internalized by clathrin-mediated endocytosis and is present in endosomes and synaptic vesicles.J Neurochem. 2003 Oct;87(1):136-46. doi: 10.1046/j.1471-4159.2003.01974.x. J Neurochem. 2003. PMID: 12969261
-
The choline transporter resurfaces: new roles for synaptic vesicles?Mol Interv. 2004 Feb;4(1):22-37. doi: 10.1124/mi.4.1.22. Mol Interv. 2004. PMID: 14993474 Review.
-
The "ins" and "outs" of the high-affinity choline transporter CHT1.J Neurochem. 2006 Apr;97(1):1-12. doi: 10.1111/j.1471-4159.2006.03695.x. Epub 2006 Mar 8. J Neurochem. 2006. PMID: 16524384 Review.
Cited by
-
Substrate-induced internalization of the high-affinity choline transporter.J Neurosci. 2011 Oct 19;31(42):14989-97. doi: 10.1523/JNEUROSCI.2983-11.2011. J Neurosci. 2011. PMID: 22016532 Free PMC article.
References
-
- Apparsundaram S, Ferguson SM, George AL, Jr, Blakely RD. Molecular cloning of a human hemicholinium-3-sensitive choline transporter. Biochem Biophys Res Commun. 2000;276(3):862–867. - PubMed
-
- Birks R, MacIntosh FC. Acetylcholine metabolism of a sympathetic ganglion. J Biochem Physiol. 1961;39:787–827.
-
- Bogan JS, Hendon N, McKee AE, Tsao T, Lodish HF. Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature. 2003;425:727–733. - PubMed
-
- Chao I. Action of electrolytes on the dorsal median nerve cord of the Limulus heart. Biol Bull Mar Biol Lab Woods Hole. 1933;64:358–381.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

