Anti-PR3 immune responses induce segmental and necrotizing glomerulonephritis
- PMID: 20015271
- PMCID: PMC2819498
- DOI: 10.1111/j.1365-2249.2009.04072.x
Anti-PR3 immune responses induce segmental and necrotizing glomerulonephritis
Abstract
Wegener's granulomatosis (WG) is a life-threatening autoimmune vasculitis that affects lungs, kidneys and other organs. A hallmark of WG is the presence of classic anti-neutrophil cytoplasmic antibodies (c-ANCA) against self-proteinase 3 (PR3). Little is known about the role of these antibodies and PR3-specific immune responses in disease development. In this study, we demonstrate that PR3-specific autoimmune responses are pathogenic in non-obese diabetic (NOD) mice with an impaired regulatory arm of the immune response. Immunization of autoimmunity prone NOD mice with rmPR3 (recombinant mouse PR3) in complete Freund's adjuvant (CFA) resulted in high levels of c-ANCA, without detectable disease development. However, when splenocytes from these immunized mice were transferred into immunodeficient NOD-severe combined immunodeficiency (SCID) mice, the recipient mice developed vasculitis and severe segmental and necrotizing glomerulonephritis. No disease developed in NOD-SCID mice that received splenocytes from the CFA-alone-immunized donors (controls), indicating that disease development depends upon PR3-specific immune responses. In contrast to the pathology observed in NOD-SCID mice, no disease was observed when splenocytes from rmPR3-immunized C57BL/6 mice were transferred into immunodeficient C57BL/6-RAG-1(-/-) mice, suggesting that complex and probably multi-genetic factors play a role in the regulation of disease development.
Figures

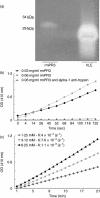

 ). The titre of anti-mPR3 antibodies in sera was monitored using enzyme-linked immunosorbent assay (ELISA) at different time-points. Control mice received CFA and IFA without antigen (
). The titre of anti-mPR3 antibodies in sera was monitored using enzyme-linked immunosorbent assay (ELISA) at different time-points. Control mice received CFA and IFA without antigen ( ). Values shown are mean ± standard deviation (n = 4), P < 0·005. (b) Anti-PR3 ELISA was performed in the presence of increasing concentrations of FLAG-peptide (0–1 mg/ml). (c) Autoantibodies are present on peripheral neutrophils/monocytes in NOD mice immunized with rmPR3 (right panel) but not CFA-alone-immunized mice (left panel). Indirect immunofluorescence staining of neutrophils with serum from NOD mice immunized with rmPR3. (d) This staining resulted in the characteristic granular cytoplasmic staining pattern. (e) Indirect immunofluorescence staining of neutrophils with serum from CFA-alone-immunized NOD mice. Original magnification of both images (d,e) ×600.
). Values shown are mean ± standard deviation (n = 4), P < 0·005. (b) Anti-PR3 ELISA was performed in the presence of increasing concentrations of FLAG-peptide (0–1 mg/ml). (c) Autoantibodies are present on peripheral neutrophils/monocytes in NOD mice immunized with rmPR3 (right panel) but not CFA-alone-immunized mice (left panel). Indirect immunofluorescence staining of neutrophils with serum from NOD mice immunized with rmPR3. (d) This staining resulted in the characteristic granular cytoplasmic staining pattern. (e) Indirect immunofluorescence staining of neutrophils with serum from CFA-alone-immunized NOD mice. Original magnification of both images (d,e) ×600.
 ) and adjuvant-alone-immunized mice (n = 2) (
) and adjuvant-alone-immunized mice (n = 2) ( ) were bled at different time-points and the anti-PR3 antibody titre was measured using enzyme-linked immunosorbent assay.
) were bled at different time-points and the anti-PR3 antibody titre was measured using enzyme-linked immunosorbent assay.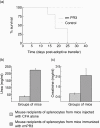
 ) or with adjuvant alone (
) or with adjuvant alone ( ).The normal range for BUN is 18–29 mg/dl and 0·2–0·8 mg/dl for creatinine in mouse serum.
).The normal range for BUN is 18–29 mg/dl and 0·2–0·8 mg/dl for creatinine in mouse serum.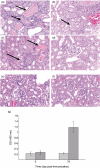
 ) (n = 3) and adjuvant-alone-immunized mice (
) (n = 3) and adjuvant-alone-immunized mice ( ) (n = 2) were bled at different time-points.
) (n = 2) were bled at different time-points.Similar articles
-
4C3 Human Monoclonal Antibody: A Proof of Concept for Non-pathogenic Proteinase 3 Anti-neutrophil Cytoplasmic Antibodies in Granulomatosis With Polyangiitis.Front Immunol. 2020 Sep 25;11:573040. doi: 10.3389/fimmu.2020.573040. eCollection 2020. Front Immunol. 2020. PMID: 33101296 Free PMC article.
-
Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system.PLoS One. 2012;7(1):e28626. doi: 10.1371/journal.pone.0028626. Epub 2012 Jan 11. PLoS One. 2012. PMID: 22247758 Free PMC article.
-
Discrimination and variable impact of ANCA binding to different surface epitopes on proteinase 3, the Wegener's autoantigen.J Autoimmun. 2010 Dec;35(4):299-308. doi: 10.1016/j.jaut.2010.06.021. J Autoimmun. 2010. PMID: 20810247 Free PMC article.
-
New advances in the pathogenesis of ANCA-associated vasculitides.Clin Exp Rheumatol. 2009 Jan-Feb;27(1 Suppl 52):S108-14. Clin Exp Rheumatol. 2009. PMID: 19646356 Review.
-
Pathogenicity of Proteinase 3-Anti-Neutrophil Cytoplasmic Antibody in Granulomatosis With Polyangiitis: Implications as Biomarker and Future Therapies.Front Immunol. 2021 Feb 18;12:571933. doi: 10.3389/fimmu.2021.571933. eCollection 2021. Front Immunol. 2021. PMID: 33679731 Free PMC article. Review.
Cited by
-
Pathogenesis and therapeutic interventions for ANCA-associated vasculitis.Nat Rev Rheumatol. 2019 Feb;15(2):91-101. doi: 10.1038/s41584-018-0145-y. Nat Rev Rheumatol. 2019. PMID: 30542206 Review.
-
Innate immune cells in the pathogenesis of primary systemic vasculitis.Rheumatol Int. 2016 Feb;36(2):169-82. doi: 10.1007/s00296-015-3367-1. Epub 2015 Sep 24. Rheumatol Int. 2016. PMID: 26403285 Review.
-
Elevated soluble Flt1 inhibits endothelial repair in PR3-ANCA-associated vasculitis.J Am Soc Nephrol. 2012 Jan;23(1):155-64. doi: 10.1681/ASN.2010080858. Epub 2011 Oct 27. J Am Soc Nephrol. 2012. PMID: 22034638 Free PMC article.
-
Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis.Annu Rev Pathol. 2013 Jan 24;8:139-60. doi: 10.1146/annurev-pathol-011811-132453. Annu Rev Pathol. 2013. PMID: 23347350 Free PMC article. Review.
-
Animal models of anti-neutrophil cytoplasmic antibody-associated vasculitis.Clin Exp Immunol. 2012 Sep;169(3):229-37. doi: 10.1111/j.1365-2249.2012.04616.x. Clin Exp Immunol. 2012. PMID: 22861362 Free PMC article. Review.
References
-
- Fahey JL, Leonard E, Churg J, Godman G. Wegener's granulomatosis. Am J Med. 1954;17:168–79. - PubMed
-
- Hewins P, Tervaert JW, Savage CO, Kallenberg CG. Is Wegener's granulomatosis an autoimmune disease? Curr Opin Rheumatol. 2000;12:3–10. - PubMed
-
- Franssen CF, Stegeman CA, Kallenberg CG, et al. Antiproteinase 3-and antimyeloperoxidase-associated vasculitis. Kidney Int. 2000;57:2195–206. - PubMed
-
- Ewert BH, Jennette JC, Falk RJ. The pathogenic role of antineutrophil cytoplasmic autoantibodies. Am J Kidney Dis. 1991;18:188–95. - PubMed
-
- Han WK, Choi HK, Roth RM, McCluskey RT, Niles JL. Serial ANCA titers: useful tool for prevention of relapses in ANCA-associated vasculitis. Kidney Int. 2003;63:1079–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

