Bimolecular fluorescence complementation: lighting up seven transmembrane domain receptor signalling networks
- PMID: 20015298
- PMCID: PMC2829200
- DOI: 10.1111/j.1476-5381.2009.00480.x
Bimolecular fluorescence complementation: lighting up seven transmembrane domain receptor signalling networks
Abstract
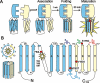
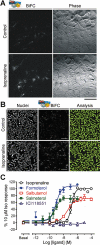
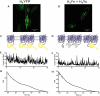
There is increasing complexity in the organization of seven transmembrane domain (7TM) receptor signalling pathways, and in the ability of their ligands to modulate and direct this signalling. Underlying these events is a network of protein interactions between the 7TM receptors themselves and associated effectors, such as G proteins and beta-arrestins. Bimolecular fluorescence complementation, or BiFC, is a technique capable of detecting these protein-protein events essential for 7TM receptor function. Fluorescent proteins, such as those from Aequorea victoria, are split into two non-fluorescent halves, which then tag the proteins under study. On association, these fragments refold and regenerate a mature fluorescent protein, producing a BiFC signal indicative of complex formation. Here, we review the experimental criteria for successful application of BiFC, considered in the context of 7TM receptor signalling events such as receptor dimerization, G protein and beta-arrestin signalling. The advantages and limitations of BiFC imaging are compared with alternative resonance energy transfer techniques. We show that the essential simplicity of the fluorescent BiFC measurement allows high-content and advanced imaging applications, and that it can probe more complex multi-protein interactions alone or in combination with resonance energy transfer. These capabilities suggest that BiFC techniques will become ever more useful in the analysis of ligand and 7TM receptor pharmacology at the molecular level of protein-protein interactions.
Figures

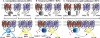
References
-
- Anderie I, Schmid A. In vivo visualization of actin dynamics and actin interactions by BiFC. Cell Biol Int. 2007;31:1131–1135. - PubMed
-
- Berglund MM, Schober DA, Statnick MA, McDonald PH, Gehlert DR. The use of bioluminescence resonance energy transfer 2 to study neuropeptide Y receptor agonist-induced β-arrestin 2 interaction. J Pharmacol Exp Ther. 2003;306:147–156. - PubMed
-
- Briddon SJ, Gandia J, Amaral OB, Ferre S, Lluis C, Franco R, et al. Plasma membrane diffusion of G protein-coupled receptor oligomers. Biochim Biophys Acta. 2008;1783:2262–2268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

