The sonic hedgehog signaling pathway is reactivated in human renal cell carcinoma and plays orchestral role in tumor growth
- PMID: 20015350
- PMCID: PMC2803450
- DOI: 10.1186/1476-4598-8-123
The sonic hedgehog signaling pathway is reactivated in human renal cell carcinoma and plays orchestral role in tumor growth
Abstract
Background: Human clear cell renal cell carcinoma (CRCC) remains resistant to therapies. Recent advances in Hypoxia Inducible Factors (HIF) molecular network led to targeted therapies, but unfortunately with only limited clinical significance. Elucidating the molecular processes involved in kidney tumorigenesis and resistance is central to the development of improved therapies, not only for kidney cancer but for many, if not all, cancer types. The oncogenic PI3K/Akt, NF-kB and MAPK pathways are critical for tumorigenesis. The sonic hedgehog (SHH) signaling pathway is crucial to normal development.
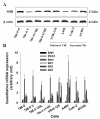
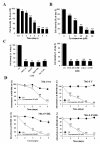
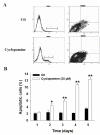
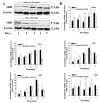
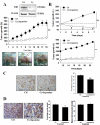
Results: By quantitative RT-PCR and immunoblot, we report that the SHH signaling pathway is constitutively reactivated in tumors independently of the von Hippel-Lindau (VHL) tumor suppressor gene expression which is inactivated in the majority of CRCC. The inhibition of the SHH signaling pathway by the specific inhibitor cyclopamine abolished CRCC cell growth as assessed by cell counting, BrdU incorporation studies, fluorescence-activated cell sorting and beta-galactosidase staining. Importantly, inhibition of the SHH pathway induced tumor regression in nude mice through inhibition of cell proliferation and neo-vascularization, and induction of apoptosis but not senescence assessed by in vivo studies, immunoblot and immunohistochemistry. Gli1, cyclin D1, Pax2, Lim1, VEGF, and TGF-beta were exclusively expressed in tumors and were shown to be regulated by SHH, as evidenced by immunoblot after SHH inhibition. Using specific inhibitors and immunoblot, the activation of the oncogenic PI3K/Akt, NF-kB and MAPK pathways was decreased by SHH inhibition.
Conclusions: These findings support targeting SHH for the treatment of CRCC and pave the way for innovative and additional investigations in a broad range of cancers.
Figures
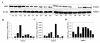

Similar articles
-
Vitamin D3 triggers antitumor activity through targeting hedgehog signaling in human renal cell carcinoma.Carcinogenesis. 2012 Nov;33(11):2084-93. doi: 10.1093/carcin/bgs255. Epub 2012 Jul 28. Carcinogenesis. 2012. PMID: 22843547
-
LIM-class homeobox gene Lim1, a novel oncogene in human renal cell carcinoma.Oncogene. 2011 Apr 14;30(15):1753-63. doi: 10.1038/onc.2010.557. Epub 2010 Dec 6. Oncogene. 2011. PMID: 21132009
-
Activation of Sonic hedgehog signaling pathway in S-type neuroblastoma cell lines.J Huazhong Univ Sci Technolog Med Sci. 2010 Jun;30(3):271-7. doi: 10.1007/s11596-010-0342-7. Epub 2010 Jun 17. J Huazhong Univ Sci Technolog Med Sci. 2010. PMID: 20556567
-
MicroRNAs Associated with Von Hippel-Lindau Pathway in Renal Cell Carcinoma: A Comprehensive Review.Int J Mol Sci. 2017 Nov 22;18(11):2495. doi: 10.3390/ijms18112495. Int J Mol Sci. 2017. PMID: 29165391 Free PMC article. Review.
-
Renal cancer: oxygen meets metabolism.Exp Cell Res. 2012 May 15;318(9):1057-67. doi: 10.1016/j.yexcr.2012.02.026. Epub 2012 Mar 3. Exp Cell Res. 2012. PMID: 22406000 Free PMC article. Review.
Cited by
-
Expression of the Sonic Hedgehog pathway components in clear cell renal cell carcinoma.Oncol Lett. 2019 Dec;18(6):5801-5810. doi: 10.3892/ol.2019.10919. Epub 2019 Sep 25. Oncol Lett. 2019. PMID: 31788053 Free PMC article.
-
TIP-B1 promotes kidney clear cell carcinoma growth and metastasis via EGFR/AKT signaling.Aging (Albany NY). 2019 Sep 27;11(18):7914-7937. doi: 10.18632/aging.102298. Epub 2019 Sep 27. Aging (Albany NY). 2019. PMID: 31562290 Free PMC article.
-
Silibinin and its 2,3-dehydro-derivative inhibit basal cell carcinoma growth via suppression of mitogenic signaling and transcription factors activation.Mol Carcinog. 2016 Jan;55(1):3-14. doi: 10.1002/mc.22253. Epub 2014 Dec 9. Mol Carcinog. 2016. PMID: 25492239 Free PMC article.
-
Multiple functional linear model for association analysis of RNA-seq with imaging.Quant Biol. 2015 Jun;3(2):90-102. doi: 10.1007/s40484-015-0048-8. Epub 2015 Aug 15. Quant Biol. 2015. PMID: 26753102 Free PMC article.
-
The Lim1 oncogene as a new therapeutic target for metastatic human renal cell carcinoma.Oncogene. 2019 Jan;38(1):60-72. doi: 10.1038/s41388-018-0413-y. Epub 2018 Aug 3. Oncogene. 2019. PMID: 30076415
References
-
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. New Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

