Association of trial registration with the results and conclusions of published trials of new oncology drugs
- PMID: 20015404
- PMCID: PMC2811705
- DOI: 10.1186/1745-6215-10-116
Association of trial registration with the results and conclusions of published trials of new oncology drugs
Abstract
Background: Registration of clinical trials has been introduced largely to reduce bias toward statistically significant results in the trial literature. Doubts remain about whether advance registration alone is an adequate measure to reduce selective publication, selective outcome reporting, and biased design. One of the first areas of medicine in which registration was widely adopted was oncology, although the bulk of registered oncology trials remain unpublished. The net influence of registration on the literature remains untested. This study compares the prevalence of favorable results and conclusions among published reports of registered and unregistered randomized controlled trials of new oncology drugs.
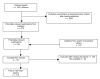
Methods: We conducted a cross-sectional study of published original research articles reporting clinical trials evaluating the efficacy of drugs newly approved for antimalignancy indications by the United States Food and Drug Administration (FDA) from 2000 through 2005. Drugs receiving first-time approval for indications in oncology were identified using the FDA web site and Thomson Centerwatch. Relevant trial reports were identified using PubMed and the Cochrane Library. Evidence of advance trial registration was obtained by a search of clinicaltrials.gov, WHO, ISRCTN, NCI-PDQ trial databases and corporate trial registries, as well as articles themselves. Data on blinding, results for primary outcomes, and author conclusions were extracted independently by two coders. Univariate and multivariate logistic regression identified associations between favorable results and conclusions and independent variables including advance registration, study design characteristics, and industry sponsorship.
Results: Of 137 original research reports from 115 distinct randomized trials assessing 25 newly approved drugs for treating cancer, the 54 publications describing data from trials registered prior to publication were as likely to report statistically significant efficacy results and reach conclusions favoring the test drug (for results, OR = 1.77; 95% CI = 0.87 to 3.61) as reports of trials not registered in advance. In multivariate analysis, reports of prior registered trials were again as likely to favor the test drug (OR = 1.29; 95% CI = 0.54 to 3.08); large sample sizes and surrogate outcome measures were statistically significant predictors of favorable efficacy results at p < 0.05. Subgroup analysis of the main reports from each trial (n = 115) similarly indicated that registered trials were as likely to report results favoring the test drug as trials not registered in advance (OR = 1.11; 95% CI = 0.44 to 2.80), and also that large trials and trials with nonstringent blinding were significantly more likely to report results favoring the test drug.
Conclusions: Trial registration alone, without a requirement for full reporting of research results, does not appear to reduce a bias toward results and conclusions favoring new drugs in the clinical trials literature. Our findings support the inclusion of full results reporting in trial registers, as well as protocols to allow assessment of whether results have been completely reported.
Figures
References
-
- Liss H. Publication bias in the pulmonary/allergy literature: effect of pharmaceutical company sponsorship. Isr Med Assoc J. 2006;8:451–454. - PubMed