Annexin A2 on lung epithelial cell surface is recognized by severe acute respiratory syndrome-associated coronavirus spike domain 2 antibodies
- PMID: 20015551
- PMCID: PMC7112629
- DOI: 10.1016/j.molimm.2009.11.019
Annexin A2 on lung epithelial cell surface is recognized by severe acute respiratory syndrome-associated coronavirus spike domain 2 antibodies
Abstract
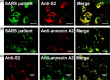
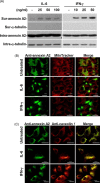
Severe acute respiratory syndrome-associated coronavirus (SARS-CoV) infection causes lung failure characterized by atypical pneumonia. We previously showed that antibodies against SARS-CoV spike domain 2 (S2) in the patient sera can cross-react with human lung epithelial cells; however, the autoantigen is not yet identified. In this study, we performed proteomic studies and identified several candidate autoantigens recognized by SARS patient sera in human lung type II epithelial cell A549. Among the candidate proteins, annexin A2, which was identified by mass spectrometry analysis and had the highest score by Mascot data search, was further characterized and investigated for its role as an autoantigen. By confocal microscopic observation, SARS patient sera and anti-S2 antibodies were co-localized on A549 cells and both of them were co-localized with anti-annexin A2 antibodies. Anti-annexin A2 antibodies bound to purified S2 proteins, and anti-S2 bound to immunoprecipitated annexin A2 from A549 cell lysate in a dose-dependent manner. Furthermore, an increased surface expression and raft-structure distribution of annexin A2 was present in A549 cells after stimulation with SARS-induced cytokines interleukin-6 and interferon-gamma. Cytokine stimulation increased the binding capability of anti-S2 antibodies to human lung epithelial cells. Together, the upregulated expression of annexin A2 by SARS-associated cytokines and the cross-reactivity of anti-SARS-CoV S2 antibodies to annexin A2 may have implications in SARS disease pathogenesis.
(c) 2009 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Antibody to severe acute respiratory syndrome (SARS)-associated coronavirus spike protein domain 2 cross-reacts with lung epithelial cells and causes cytotoxicity.Clin Exp Immunol. 2005 Sep;141(3):500-8. doi: 10.1111/j.1365-2249.2005.02864.x. Clin Exp Immunol. 2005. PMID: 16045740 Free PMC article.
-
Identification of an antigenic determinant on the S2 domain of the severe acute respiratory syndrome coronavirus spike glycoprotein capable of inducing neutralizing antibodies.J Virol. 2004 Jul;78(13):6938-45. doi: 10.1128/JVI.78.13.6938-6945.2004. J Virol. 2004. PMID: 15194770 Free PMC article.
-
T-cell epitopes in severe acute respiratory syndrome (SARS) coronavirus spike protein elicit a specific T-cell immune response in patients who recover from SARS.J Virol. 2004 Jun;78(11):5612-8. doi: 10.1128/JVI.78.11.5612-5618.2004. J Virol. 2004. PMID: 15140958 Free PMC article.
-
Neutralizing human monoclonal antibodies to severe acute respiratory syndrome coronavirus: target, mechanism of action, and therapeutic potential.Rev Med Virol. 2012 Jan;22(1):2-17. doi: 10.1002/rmv.706. Epub 2011 Sep 8. Rev Med Virol. 2012. PMID: 21905149 Free PMC article. Review.
-
Severe acute respiratory syndrome coronavirus entry into host cells: Opportunities for therapeutic intervention.Med Res Rev. 2006 Jul;26(4):414-33. doi: 10.1002/med.20055. Med Res Rev. 2006. PMID: 16521129 Free PMC article. Review.
Cited by
-
A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity.Nat Commun. 2020 Sep 17;11(1):4704. doi: 10.1038/s41467-020-18450-4. Nat Commun. 2020. PMID: 32943637 Free PMC article.
-
Annexin A2 in Fibrinolysis, Inflammation and Fibrosis.Int J Mol Sci. 2021 Jun 25;22(13):6836. doi: 10.3390/ijms22136836. Int J Mol Sci. 2021. PMID: 34202091 Free PMC article. Review.
-
Annexin A2 as a target endothelial cell membrane autoantigen in Behçet's disease.Sci Rep. 2015 Feb 2;5:8162. doi: 10.1038/srep08162. Sci Rep. 2015. PMID: 25641213 Free PMC article.
-
Annexin A2: the missing piece in the puzzle of pathogen-induced damage.Virulence. 2023 Dec;14(1):2237222. doi: 10.1080/21505594.2023.2237222. Virulence. 2023. PMID: 37482693 Free PMC article. Review.
-
A proteomic perspective and involvement of cytokines in SARS-CoV-2 infection.PLoS One. 2023 Jan 6;18(1):e0279998. doi: 10.1371/journal.pone.0279998. eCollection 2023. PLoS One. 2023. PMID: 36608055 Free PMC article.
References
-
- Barzilai O., Ram M., Shoenfeld Y. Viral infection can induce the production of autoantibodies. Curr. Opin. Rheumatol. 2007;19:636–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

