Expansion of beta-cell mass in response to pregnancy
- PMID: 20015659
- PMCID: PMC3627215
- DOI: 10.1016/j.tem.2009.11.001
Expansion of beta-cell mass in response to pregnancy
Abstract
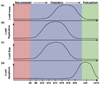
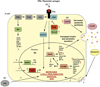
Inadequate beta-cell mass can lead to insulin insufficiency and diabetes. During times of prolonged metabolic demand for insulin, the endocrine pancreas can respond by increasing beta-cell mass, both by increasing cell size and by changing the balance between beta-cell proliferation and apoptosis. In this paper, we review recent advances in our understanding of the mechanisms that control the adaptive expansion of beta-cell mass, focusing on the islet's response to pregnancy, a physiological state of insulin resistance. Functional characterization of factors controlling both beta-cell proliferation and survival might not only lead to the development of successful therapeutic strategies to enhance the response of the beta-cell to increased metabolic loads, but also improve islet transplantation regimens.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures


References
-
- Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm. Metab. Res. 1997;29:301–307. - PubMed
-
- Kloppel G, et al. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv. Synth. Pathol. Res. 1985;4:110–125. - PubMed
-
- Bruning JC, et al. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561–572. - PubMed
-
- Zimmet P, et al. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

