Thyroid hormone crosstalk with nuclear receptor signaling in metabolic regulation
- PMID: 20015660
- PMCID: PMC2831161
- DOI: 10.1016/j.tem.2009.11.004
Thyroid hormone crosstalk with nuclear receptor signaling in metabolic regulation
Abstract
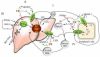
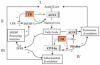
Thyroid hormone influences diverse metabolic pathways important in lipid and glucose metabolism, lipolysis and regulation of body weight. Recently, it has been recognized that thyroid hormone receptor interacts with transcription factors that predominantly respond to nutrient signals including the peroxisome proliferator-activated receptors, liver X receptor and others. Crosstalk between thyroid hormone signaling and these nutrient responsive factors occurs through a variety of mechanisms: competition for retinoid X receptor heterodimer partners, DNA binding sites and transcriptional cofactors. This review focuses on the mechanisms of interaction of thyroid hormone signaling with other metabolic pathways and the importance of understanding these interactions to develop therapeutic agents for treatment of metabolic disorders, such as dyslipidemias, obesity and diabetes.
Published by Elsevier Ltd.
Figures
References
-
- Oetting A, Yen PM. New insights into thyroid hormone action. Best Pract. Res. Clin. Endocrinol. Metab. 2007;21:193–208. - PubMed
-
- Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–1735. - PubMed
-
- Hollenberg AN. The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid. 2008;18:131–139. - PubMed
-
- Fox CS, et al. Relation of thyroid function to body weight. Arch. Intern. Med. 2008;168:587–592. - PubMed
-
- Brenta G, et al. Potential therapeutic applications of thyroid hormone analogs. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:632–640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

