Glucocorticoid receptor over-expression promotes human small cell lung cancer apoptosis in vivo and thereby slows tumor growth
- PMID: 20015838
- PMCID: PMC2828806
- DOI: 10.1677/ERC-09-0241
Glucocorticoid receptor over-expression promotes human small cell lung cancer apoptosis in vivo and thereby slows tumor growth
Abstract
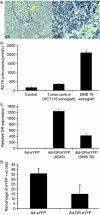
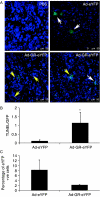
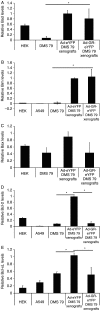
Small cell lung cancer (SCLC) is an aggressive tumor, associated with ectopic ACTH syndrome. We have shown that SCLC cells are glucocorticoid receptor (GR) deficient, and that restoration of GR expression confers glucocorticoid sensitivity and induces apoptosis in vitro. To determine the effects of GR expression in vivo, we characterized a mouse SCLC xenograft model that secretes ACTH precursor peptides, and so drives high circulating corticosterone concentrations (analogous to the ectopic ACTH syndrome). Infection of SCLC xenografts with GR-expressing adenovirus significantly slowed tumor growth compared with control virus infection. Time to fourfold initial tumor volume increased from a median of 9 days to 16 days (P=0.05; n=7 per group). Post-mortem analysis of GR-expressing tumors revealed a threefold increase in apoptotic (TUNEL positive) cells (P<0.01). Infection with the GR-expressing adenovirus caused a significant reduction in Bcl-2 and Bcl-xL transcripts. Furthermore, in both the GR-expressing adenovirus-infected cells and tumors, a significant number of uninfected cells underwent apoptosis, supporting a bystander cell killing effect. Therefore, GR expression is pro-apoptotic for human SCLCs in vivo, as well as in vitro, suggesting that loss of GR confers a survival advantage to SCLCs.
Figures

Similar articles
-
Loss of glucocorticoid receptor expression by DNA methylation prevents glucocorticoid induced apoptosis in human small cell lung cancer cells.PLoS One. 2011;6(10):e24839. doi: 10.1371/journal.pone.0024839. Epub 2011 Oct 3. PLoS One. 2011. PMID: 21984896 Free PMC article.
-
Glucocorticoid receptor-mediated apoptosis in small-cell lung cancer requires interaction with BCL2.Endocr Relat Cancer. 2013 Oct 14;20(6):785-95. doi: 10.1530/ERC-13-0402. Print 2013 Dec. Endocr Relat Cancer. 2013. PMID: 24036132
-
The N-terminal transactivation domain of the glucocorticoid receptor mediates apoptosis of human small cell lung cancer cells.Genes Chromosomes Cancer. 2014 Dec;53(12):999-1007. doi: 10.1002/gcc.22209. Epub 2014 Aug 13. Genes Chromosomes Cancer. 2014. PMID: 25116573
-
Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene.Cancer Res. 2001 Apr 15;61(8):3330-8. Cancer Res. 2001. PMID: 11309289
-
Glucocorticoid receptor overexpression exerts an antisurvival effect on human small cell lung cancer cells.Oncogene. 2007 Nov 1;26(50):7111-21. doi: 10.1038/sj.onc.1210524. Epub 2007 May 14. Oncogene. 2007. PMID: 17496926
Cited by
-
A dual role for glucocorticoid-induced leucine zipper in glucocorticoid function: tumor growth promotion or suppression?Cell Death Dis. 2018 May 1;9(5):463. doi: 10.1038/s41419-018-0558-1. Cell Death Dis. 2018. PMID: 29695779 Free PMC article. Review.
-
The tobacco carcinogen NNK drives accumulation of DNMT1 at the GR promoter thereby reducing GR expression in untransformed lung fibroblasts.Sci Rep. 2018 Mar 20;8(1):4903. doi: 10.1038/s41598-018-23309-2. Sci Rep. 2018. PMID: 29559689 Free PMC article.
-
Targeting Nuclear Receptors in Lung Cancer-Novel Therapeutic Prospects.Pharmaceuticals (Basel). 2022 May 18;15(5):624. doi: 10.3390/ph15050624. Pharmaceuticals (Basel). 2022. PMID: 35631448 Free PMC article. Review.
-
BAD overexpression inhibits cell growth and induces apoptosis via mitochondrial-dependent pathway in non-small cell lung cancer.Cancer Cell Int. 2013 Jun 1;13(1):53. doi: 10.1186/1475-2867-13-53. Cancer Cell Int. 2013. PMID: 23725574 Free PMC article.
-
Glucocorticoid receptor regulates accurate chromosome segregation and is associated with malignancy.Proc Natl Acad Sci U S A. 2015 Apr 28;112(17):5479-84. doi: 10.1073/pnas.1411356112. Epub 2015 Apr 6. Proc Natl Acad Sci U S A. 2015. PMID: 25847991 Free PMC article.
References
-
- Crosby SR, Stewart MF, Ratcliffe JG, White A. Direct measurement of the precursors of adrenocorticotropin in human plasma by two-site immunoradiometric assay. Journal of Clinical Endocrinology and Metabolism. 1988;67:1272–1277. - PubMed
-
- Donn R, Berry A, Stevens A, Farrow S, Betts J, Stevens R, Clayton C, Wang J, Warnock L, Worthington J, et al. Use of gene expression profiling to identify a novel glucocorticoid sensitivity determining gene, BMPRII. FASEB Journal. 2007;21:402–414. - PubMed
-
- Herr I, Gassler N, Friess H, Buchler MW. Regulation of differential pro- and anti-apoptotic signaling by glucocorticoids. Apoptosis. 2007;12:271–291. - PubMed
-
- Hofmann J, Kaiser U, Maasberg M, Havemann K. Glucocorticoid receptors and growth inhibitory effects of dexamethasone in human lung cancer cell lines. European Journal of Cancer. 1995;31A:2053–2058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

