Mouse mesenchymal stem cells can support human hematopoiesis both in vitro and in vivo: the crucial role of neural cell adhesion molecule
- PMID: 20015889
- PMCID: PMC2878784
- DOI: 10.3324/haematol.2009.013151
Mouse mesenchymal stem cells can support human hematopoiesis both in vitro and in vivo: the crucial role of neural cell adhesion molecule
Abstract
Background: We previously established a mesenchymal stem cell line (FMS/PA6-P) from the bone marrow adherent cells of fetal mice. The cell line expresses a higher level of neural cell adhesion molecule and shows greater hematopoiesis-supporting capacity in mice than other murine stromal cell lines.
Design and methods: Since there is 94% homology between human and murine neural cell adhesion molecule, we examined whether FMS/PA6-P cells support human hematopoiesis and whether neural cell adhesion molecules expressed on FMS/PA6-P cells contribute greatly to the human hematopoiesis-supporting ability of the cell line.
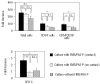
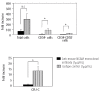
Results: When lineage-negative cord blood mononuclear cells were co-cultured on the FMS/PA6-P cells, a significantly greater hematopoietic stem cell-enriched population (CD34(+)CD38(-) cells) was obtained than in the culture without the FMS/PA6-P cells. Moreover, when lineage-negative cord blood mononuclear cells were cultured on FMS/PA6-P cells and transplanted into SCID mice, a significantly larger proportion of human CD45(+) cells and CD34(+)CD38(-) cells were detected in the bone marrow of SCID mice than in the bone marrow of SCID mice that had received lineage-negative cord blood mononuclear cells cultured without FMS/PA6-P cells. Furthermore, we found that direct cell-to-cell contact between the lineage-negative cord blood mononuclear cells and the FMS/PA6-P cells was essential for the maximum expansion of the mononuclear cells. The addition of anti-mouse neural cell adhesion molecule antibody to the culture significantly inhibited their contact and the proliferation of lineage-negative cord blood mononuclear cells.
Conclusions: These findings suggest that neural cell adhesion molecules expressed on FMS/PA6-P cells play a crucial role in the human hematopoiesis-supporting ability of the cell line.
Figures


Similar articles
-
Neural cell adhesion molecule contributes to hemopoiesis-supporting capacity of stromal cell lines.Stem Cells. 2005 Oct;23(9):1389-99. doi: 10.1634/stemcells.2004-0343. Epub 2005 Jul 28. Stem Cells. 2005. PMID: 16051987
-
Characterization of mesenchymal stem cells isolated from mouse fetal bone marrow.Stem Cells. 2006 Mar;24(3):482-93. doi: 10.1634/stemcells.2005-0219. Epub 2005 Sep 22. Stem Cells. 2006. PMID: 16179426
-
Mimicking the functional hematopoietic stem cell niche in vitro: recapitulation of marrow physiology by hydrogel-based three-dimensional cultures of mesenchymal stromal cells.Haematologica. 2012 May;97(5):651-60. doi: 10.3324/haematol.2011.050500. Epub 2011 Nov 4. Haematologica. 2012. PMID: 22058199 Free PMC article.
-
Mesenchymal progenitors and the osteoblast lineage in bone marrow hematopoietic niches.Curr Osteoporos Rep. 2014 Mar;12(1):22-32. doi: 10.1007/s11914-014-0190-7. Curr Osteoporos Rep. 2014. PMID: 24477415 Free PMC article. Review.
-
Regulation of hematopoiesis by microvascular endothelium.Leuk Lymphoma. 1997 Nov;27(5-6):375-86. doi: 10.3109/10428199709058305. Leuk Lymphoma. 1997. PMID: 9477120 Review.
Cited by
-
Mesenchymal stem cells secreting angiopoietin-like-5 support efficient expansion of human hematopoietic stem cells without compromising their repopulating potential.Stem Cells Dev. 2011 Aug;20(8):1371-81. doi: 10.1089/scd.2010.0456. Epub 2011 Jan 31. Stem Cells Dev. 2011. PMID: 21142526 Free PMC article.
-
Expression of neural cell adhesion molecule and polysialic acid in human bone marrow-derived mesenchymal stromal cells.Stem Cell Res Ther. 2016 Aug 15;7(1):113. doi: 10.1186/s13287-016-0373-5. Stem Cell Res Ther. 2016. PMID: 27528376 Free PMC article.
-
Gene-expression and in vitro function of mesenchymal stromal cells are affected in juvenile myelomonocytic leukemia.Haematologica. 2015 Nov;100(11):1434-41. doi: 10.3324/haematol.2015.126938. Epub 2015 Aug 20. Haematologica. 2015. PMID: 26294732 Free PMC article. Clinical Trial.
-
The efficacy of topical insulin application on rat model with burn wounds treated with adipose-derived stem cells.Int J Burns Trauma. 2020 Dec 15;10(6):296-306. eCollection 2020. Int J Burns Trauma. 2020. PMID: 33500841 Free PMC article.
-
Human olfactory mucosa multipotent mesenchymal stromal cells promote survival, proliferation, and differentiation of human hematopoietic cells.Stem Cells Dev. 2012 Nov 20;21(17):3187-96. doi: 10.1089/scd.2012.0084. Epub 2012 May 18. Stem Cells Dev. 2012. PMID: 22471939 Free PMC article.
References
-
- Gluckman E. Current status of umbilical cord blood hematopoietic stem cell transplantation. Exp Hematol. 2000;28(11):1197–205. - PubMed
-
- Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, et al. Outcomes among 562 recipients of placental blood transplants from unrelated donors. N Engl J Med. 1998;339(22):1565–77. - PubMed
-
- Rocha V, Wagner JE, Jr, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling: Eurocord and International Bone Marrow Transplant Registry Working Committeebon Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342(25):1846–54. - PubMed
-
- Locatelli F, Rocha V, Chastang C, Arcese W, Michel G, Abecasis M, et al. Factors associated with outcome after cord blood transplantation in children with acute leukemia: Eurocord-Cord Blood Transplant Group. Blood. 1999;93(11):3662–71. - PubMed
-
- Jaroscak J, Goltry K, Smith A, Waters-Pick B, Martin PL, Driscoll TA, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: results of a phase 1 trial using the AastromReplicell System. Blood. 2003;101(12):5061–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

