Topical intranasal corticosteroids in 4-11 year old children with persistent bilateral otitis media with effusion in primary care: double blind randomised placebo controlled trial
- PMID: 20015903
- PMCID: PMC2795136
- DOI: 10.1136/bmj.b4984
Topical intranasal corticosteroids in 4-11 year old children with persistent bilateral otitis media with effusion in primary care: double blind randomised placebo controlled trial
Abstract
Objective: To determine the clinical effectiveness of topical intranasal corticosteroids in children with bilateral otitis media with effusion.
Design: Double blind randomised placebo controlled trial.
Setting: 76 Medical Research Council General Practice Research Framework practices throughout the United Kingdom, between 2004 and 2007.
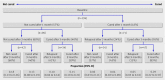
Participants: 217 children aged 4-11 years who had at least one practice recorded episode of otitis media or a related ear problem in the previous 12 months, and with bilateral otitis media with effusion confirmed by a research nurse using otoscopy plus micro-tympanometry (B/B or B/C2, modified Jerger types).
Intervention: Mometasone furoate 50 microg or placebo spray given once daily into each nostril for three months.
Main outcome measures: Proportions of children cured of bilateral otitis media with effusion assessed with tympanometry (C1 or A type) at one month (primary end point), three months, and nine months; adverse events; three month diary symptoms. Results 41% (39/96) of the topical steroid group and 45% (44/98) of the placebo group were cured in one or both ears at one month (difference favouring placebo 4.3% (95% confidence interval -9.3% to 18.1%). Poisson regression was done with adjustment for four pre-specified covariates (clinical severity, P=0.003; atopy, P=0.67; age, P=0.92; season, P=0.71). The adjusted relative risk at one month was 0.97 (95% confidence interval 0.74 to 1.26). At three months, 58% of the topical steroid group and 52% of the placebo group were cured (relative risk 1.23, 0.84 to 1.80). Diary symptoms did not differ between the two groups, and no significant harms were reported.
Conclusions: Topical steroids are unlikely to be an effective treatment for otitis media with effusion in general practice. High rates of natural resolution occurred by 1-3 months.
Trial registration: Current Controlled Trials ISRCTN38988331; National Research Register NO575123823; MREC 03/11/073.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
Topical intranasal corticosteroids for otitis media with effusion in primary care.BMJ. 2010 Jan 7;340:b5380. doi: 10.1136/bmj.b5380. BMJ. 2010. PMID: 20056694 No abstract available.
-
Topical intranasal steroids do not benefit children with persistent middle ear effusion.J Pediatr. 2010 Jul;157(1):171-2. doi: 10.1016/j.jpeds.2010.05.009. J Pediatr. 2010. PMID: 20547270 No abstract available.
References
-
- Stool SE, Berg AO, Berman S, Carney CJ, Cooley JR, Culpepper L, et al. Otitis media with effusion in children. 1994. www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hsarchive&part=A34095.
-
- Department of Health. Trends in children’s surgery 1994-2005: evidence from hospital episode statistics data. Stationery Office, 2007 (available at www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsStat...).
-
- Haggard MP, Smith SC. Impact of otitis media on child quality of life. In: Rosenfeld RM, Bluestone CD, eds. Evidence based otitis media. BC Decker, 1999:375-98.
-
- Zielhuis GA, Rach GH, Broek PV. Screening for otitis media with effusion in preschool children. Lancet 1989;1:311-4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous