Extracellular glutathione peroxidase (Gpx3) binds specifically to basement membranes of mouse renal cortex tubule cells
- PMID: 20015939
- PMCID: PMC2867408
- DOI: 10.1152/ajprenal.00662.2009
Extracellular glutathione peroxidase (Gpx3) binds specifically to basement membranes of mouse renal cortex tubule cells
Abstract
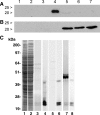
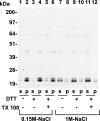
Glutathione peroxidase-3 (Gpx3), also known as plasma or extracellular glutathione peroxidase, is a selenoprotein secreted primarily by kidney proximal convoluted tubule cells. In this study Gpx3(-/-) mice have been produced and immunocytochemical techniques have been developed to investigate Gpx3 metabolism. Gpx3(-/-) mice maintained the same whole-body content and urinary excretion of selenium as did Gpx3(+/+) mice. They tolerated selenium deficiency without observable ill effects. The simultaneous knockout of Gpx3 and selenoprotein P revealed that these two selenoproteins account for >97% of plasma selenium. Immunocytochemistry experiments demonstrated that Gpx3 binds selectively, both in vivo and in vitro, to basement membranes of renal cortical proximal and distal convoluted tubules. Based on calculations using selenium content, the kidney pool of Gpx3 is over twice as large as the plasma pool. These data indicate that Gpx3 does not serve in the regulation of selenium metabolism. The specific binding of a large pool of Gpx3 to basement membranes in the kidney cortex strongly suggests a need for glutathione peroxidase activity in the cortical peritubular space.
Figures





Similar articles
-
High-resolution imaging of selenium in kidneys: a localized selenium pool associated with glutathione peroxidase 3.Antioxid Redox Signal. 2012 Feb 1;16(3):185-92. doi: 10.1089/ars.2011.3997. Epub 2011 Nov 22. Antioxid Redox Signal. 2012. PMID: 21854231 Free PMC article.
-
Impact of Glutathione Peroxidase-1 (Gpx1) Genotype on Selenoenzyme and Transcript Expression When Repleting Selenium-Deficient Mice.Biol Trace Elem Res. 2018 Nov;186(1):174-184. doi: 10.1007/s12011-018-1281-6. Epub 2018 Mar 3. Biol Trace Elem Res. 2018. PMID: 29502249
-
Glutathione peroxidase-3 produced by the kidney binds to a population of basement membranes in the gastrointestinal tract and in other tissues.Am J Physiol Gastrointest Liver Physiol. 2011 Jul;301(1):G32-8. doi: 10.1152/ajpgi.00064.2011. Epub 2011 Apr 14. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21493731 Free PMC article.
-
Selenium, selenoproteins and human health: a review.Public Health Nutr. 2001 Apr;4(2B):593-9. doi: 10.1079/phn2001143. Public Health Nutr. 2001. PMID: 11683552 Review.
-
Recent developments in trace element metabolism and function: newer roles of selenium in nutrition.J Nutr. 1989 Jul;119(7):1051-4. doi: 10.1093/jn/119.7.1051. J Nutr. 1989. PMID: 2666600 Review.
Cited by
-
Developmental expression of plasma glutathione peroxidase during mouse organogenesis.J Mol Histol. 2011 Dec;42(6):545-56. doi: 10.1007/s10735-011-9362-2. Epub 2011 Sep 27. J Mol Histol. 2011. PMID: 21948268
-
The Roles of Glutathione Peroxidases during Embryo Development.Front Mol Neurosci. 2011 Jul 28;4:12. doi: 10.3389/fnmol.2011.00012. eCollection 2011. Front Mol Neurosci. 2011. PMID: 21847368 Free PMC article.
-
Maternal-fetal transfer of selenium in the mouse.FASEB J. 2013 Aug;27(8):3249-56. doi: 10.1096/fj.13-231852. Epub 2013 May 7. FASEB J. 2013. PMID: 23651543 Free PMC article.
-
The aging mouse lens transcriptome.Exp Eye Res. 2021 Aug;209:108663. doi: 10.1016/j.exer.2021.108663. Epub 2021 Jun 11. Exp Eye Res. 2021. PMID: 34119483 Free PMC article.
-
Tolerance to Selenoprotein Loss Differs between Human and Mouse.Mol Biol Evol. 2020 Feb 1;37(2):341-354. doi: 10.1093/molbev/msz218. Mol Biol Evol. 2020. PMID: 31560400 Free PMC article.
References
-
- Avissar N, Ornt DB, Yagil Y, Horowitz S, Watkins RH, Kerl EA, Takahashi K, Palmer IS, Cohen HJ. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am J Physiol Cell Physiol 266: C367–C375, 1994 - PubMed
-
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340: 783–795, 2004 - PubMed
-
- Burk RF, Hill KE, Motley AK. Plasma selenium in specific and non-specific forms. Biofactors 14: 107–114, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

