Inhibition of sterol biosynthesis reduces tombusvirus replication in yeast and plants
- PMID: 20015981
- PMCID: PMC2820916
- DOI: 10.1128/JVI.02003-09
Inhibition of sterol biosynthesis reduces tombusvirus replication in yeast and plants
Abstract
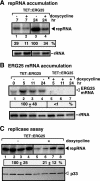
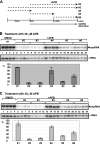
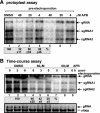
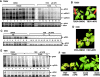
The replication of plus-strand RNA viruses depends on subcellular membranes. Recent genome-wide screens have revealed that the sterol biosynthesis genes ERG25 and ERG4 affected the replication of Tomato bushy stunt virus (TBSV) in a yeast model host. To further our understanding of the role of sterols in TBSV replication, we demonstrate that the downregulation of ERG25 or the inhibition of the activity of Erg25p with an inhibitor (6-amino-2-n-pentylthiobenzothiazole; APB) leads to a 3- to 5-fold reduction in TBSV replication in yeast. In addition, the sterol biosynthesis inhibitor lovastatin reduced TBSV replication by 4-fold, confirming the importance of sterols in viral replication. We also show reduced stability for the p92(pol) viral replication protein as well as a decrease in the in vitro activity of the tombusvirus replicase when isolated from APB-treated yeast. Moreover, APB treatment inhibits TBSV RNA accumulation in plant protoplasts and in Nicotiana benthamiana leaves. The inhibitory effect of APB on TBSV replication can be complemented by exogenous stigmasterol, the main plant sterol, suggesting that sterols are required for TBSV replication. The silencing of SMO1 and SMO2 genes, which are orthologs of ERG25, in N. benthamiana reduced TBSV RNA accumulation but had a lesser inhibitory effect on the unrelated Tobacco mosaic virus, suggesting that various viruses show different levels of dependence on sterol biosynthesis for their replication.
Figures



References
-
- Aizaki, H., K. J. Lee, V. M. Sung, H. Ishiko, and M. M. Lai. 2004. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology 324:450-461. - PubMed
-
- Bloch, K. 1992. Sterol molecule: structure, biosynthesis, and function. Steroids 57:378-383. - PubMed
-
- Bloch, K. E. 1983. Sterol structure and membrane function. CRC Crit. Rev. Biochem. 14:47-92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

