Comparative immunogenicity of subtype a Human Immunodeficiency Virus type 1 envelope exhibiting differential exposure of conserved neutralization epitopes
- PMID: 20015987
- PMCID: PMC2820908
- DOI: 10.1128/JVI.01687-09
Comparative immunogenicity of subtype a Human Immunodeficiency Virus type 1 envelope exhibiting differential exposure of conserved neutralization epitopes
Abstract
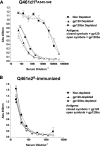
Development of broadly cross-reactive neutralizing antibodies (NAbs) remains a major goal of HIV-1 vaccine development, but most candidate envelope immunogens have had limited ability to cross-neutralize heterologous strains. To evaluate the immunogenicity of subtype A variants of HIV-1, rabbits were immunized with pairs of closely related subtype A envelopes from the same individual. In each immunogen pair, one variant was readily neutralized by a variety of monoclonal antibodies and plasma antibodies, while the other was neutralization resistant, suggesting differences in the exposures of key epitopes. The breadth of the antibody response was evaluated against subtype A, B, C, and D variants of HIV-1. The specificity of the immunogen-derived neutralizing antibody response was also compared to that of the infected individuals from whom these variants were cloned. None of the immunogens produced broad neutralizing antibodies in immunized animals, and most of the neutralizing antibodies were directed to the variable loops, particularly the V3 loop. No detectable antibodies to either of the potentially exposed conserved epitopes, the membrane proximal external region, or the CD4 binding site were found with immunized rabbits. In contrast, relatively little of the neutralizing activity within the plasma samples of the infected individuals was directed to linear epitopes within the variable loops. These data indicate that immunogens designed to expose conserved regions did not enhance generation of broadly neutralizing antibodies in comparison with the immunogens that failed to expose those regions using this immunization approach.
Figures

Similar articles
-
Conformational Epitope-Specific Broadly Neutralizing Plasma Antibodies Obtained from an HIV-1 Clade C-Infected Elite Neutralizer Mediate Autologous Virus Escape through Mutations in the V1 Loop.J Virol. 2016 Jan 13;90(7):3446-57. doi: 10.1128/JVI.03090-15. J Virol. 2016. PMID: 26763999 Free PMC article.
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Alterations in the immunogenic properties of soluble trimeric human immunodeficiency virus type 1 envelope proteins induced by deletion or heterologous substitutions of the V1 loop.J Virol. 2010 Oct;84(19):9932-46. doi: 10.1128/JVI.00868-10. Epub 2010 Jul 21. J Virol. 2010. PMID: 20660181 Free PMC article.
-
Roles of HIV-1 Env variable regions in viral neutralization and vaccine development.Curr HIV Res. 2007 Nov;5(6):542-53. doi: 10.2174/157016207782418470. Curr HIV Res. 2007. PMID: 18045110 Review.
-
Challenges for structure-based HIV vaccine design.Curr Opin HIV AIDS. 2009 Sep;4(5):431-40. doi: 10.1097/COH.0b013e32832e6184. Curr Opin HIV AIDS. 2009. PMID: 20048708 Review.
Cited by
-
Hyperimmune bovine colostrum as a low-cost, large-scale source of antibodies with broad neutralizing activity for HIV-1 envelope with potential use in microbicides.Antimicrob Agents Chemother. 2012 Aug;56(8):4310-9. doi: 10.1128/AAC.00453-12. Epub 2012 Jun 4. Antimicrob Agents Chemother. 2012. PMID: 22664963 Free PMC article.
-
The role of amino acid changes in the human immunodeficiency virus type 1 transmembrane domain in antibody binding and neutralization.Virology. 2011 Dec 20;421(2):235-44. doi: 10.1016/j.virol.2011.09.032. Epub 2011 Oct 24. Virology. 2011. PMID: 22029936 Free PMC article.
-
Binding interactions between soluble HIV envelope glycoproteins and quaternary-structure-specific monoclonal antibodies PG9 and PG16.J Virol. 2011 Jul;85(14):7095-107. doi: 10.1128/JVI.00411-11. Epub 2011 May 4. J Virol. 2011. PMID: 21543501 Free PMC article.
-
HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads.PLoS Pathog. 2012;8(6):e1002739. doi: 10.1371/journal.ppat.1002739. Epub 2012 Jun 14. PLoS Pathog. 2012. PMID: 22719248 Free PMC article.
-
B cell clonal lineage alterations upon recombinant HIV-1 envelope immunization of rhesus macaques.PLoS Pathog. 2018 Jun 22;14(6):e1007120. doi: 10.1371/journal.ppat.1007120. eCollection 2018 Jun. PLoS Pathog. 2018. PMID: 29933399 Free PMC article.
References
-
- Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. - PMC - PubMed
-
- Beddows, S., M. Franti, A. K. Dey, M. Kirschner, S. P. Iyer, D. C. Fisch, T. Ketas, E. Yuste, R. C. Desrosiers, P. J. Klasse, P. J. Maddon, W. C. Olson, and J. P. Moore. 2007. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology 360:329-340. - PubMed
-
- Beddows, S., N. Schulke, M. Kirschner, K. Barnes, M. Franti, E. Michael, T. Ketas, R. W. Sanders, P. J. Maddon, W. C. Olson, and J. P. Moore. 2005. Evaluating the immunogenicity of a disulfide-stabilized, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J. Virol. 79:8812-8827. - PMC - PubMed
-
- Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

