Arcuate nucleus proopiomelanocortin neurons mediate the acute anorectic actions of leukemia inhibitory factor via gp130
- PMID: 20016025
- PMCID: PMC2817620
- DOI: 10.1210/en.2009-1135
Arcuate nucleus proopiomelanocortin neurons mediate the acute anorectic actions of leukemia inhibitory factor via gp130
Abstract
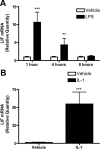
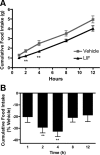
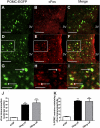
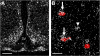
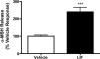
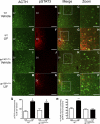
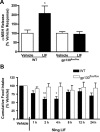
The proinflammatory cytokine leukemia inhibitory factor (LIF) is induced in disease states and is known to inhibit food intake when administered centrally. However, the neural pathways underlying this effect are not well understood. We demonstrate that LIF acutely inhibits food intake by directly activating pro-opiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus. We show that arcuate POMC neurons express the LIF-R, and that LIF stimulates the release of the anorexigenic peptide, alpha-MSH from ex vivo hypothalami. Transgenic mice lacking gp130, the signal transducing subunit of the LIF-R complex, specifically in POMC neurons fail to respond to LIF. Furthermore, LIF does not stimulate the release of alpha-MSH from the transgenic hypothalamic explants. These findings indicate that POMC neurons mediate the acute anorectic actions of central LIF administration and provide a mechanistic link between inflammation and food intake.
Figures
Comment in
-
Whatever way weight goes, inflammation shows.Endocrinology. 2010 Mar;151(3):846-8. doi: 10.1210/en.2009-1470. Endocrinology. 2010. PMID: 20172971 No abstract available.
Similar articles
-
gp130 signaling in proopiomelanocortin neurons mediates the acute anorectic response to centrally applied ciliary neurotrophic factor.Proc Natl Acad Sci U S A. 2006 Jul 11;103(28):10707-12. doi: 10.1073/pnas.0600425103. Epub 2006 Jul 3. Proc Natl Acad Sci U S A. 2006. PMID: 16818888 Free PMC article.
-
The orexigenic activity of the hypothalamic neuropeptide 26RFa is mediated by the neuropeptide Y and proopiomelanocortin neurons of the arcuate nucleus.Endocrinology. 2009 May;150(5):2342-50. doi: 10.1210/en.2008-1432. Epub 2009 Jan 22. Endocrinology. 2009. PMID: 19164468
-
Regulation of central melanocortin signaling by interleukin-1 beta.Endocrinology. 2007 Sep;148(9):4217-25. doi: 10.1210/en.2007-0017. Epub 2007 May 24. Endocrinology. 2007. PMID: 17525125
-
Regulation of prohormone convertase 1 (PC1) by gp130-related cytokines.Mol Cell Endocrinol. 1999 Dec 20;158(1-2):143-52. doi: 10.1016/s0303-7207(99)00168-9. Mol Cell Endocrinol. 1999. PMID: 10630414
-
The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis.Recent Prog Horm Res. 2004;59:395-408. doi: 10.1210/rp.59.1.395. Recent Prog Horm Res. 2004. PMID: 14749511 Review.
Cited by
-
A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis.Cells. 2019 Jul 18;8(7):738. doi: 10.3390/cells8070738. Cells. 2019. PMID: 31323874 Free PMC article. Review.
-
Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus.Nat Neurosci. 2017 Jul;20(7):934-942. doi: 10.1038/nn.4574. Epub 2017 Jun 5. Nat Neurosci. 2017. PMID: 28581479 Free PMC article.
-
Neuroendocrine and Behavioral Consequences of Hyperglycemia in Cancer.Endocrinology. 2020 May 1;161(5):bqaa047. doi: 10.1210/endocr/bqaa047. Endocrinology. 2020. PMID: 32193527 Free PMC article. Review.
-
Mechanisms of metabolic dysfunction in cancer-associated cachexia.Genes Dev. 2016 Mar 1;30(5):489-501. doi: 10.1101/gad.276733.115. Genes Dev. 2016. PMID: 26944676 Free PMC article. Review.
-
Pathophysiology of anorexia in the cancer cachexia syndrome.J Cachexia Sarcopenia Muscle. 2015 Dec;6(4):287-302. doi: 10.1002/jcsm.12059. Epub 2015 Oct 27. J Cachexia Sarcopenia Muscle. 2015. PMID: 26675762 Free PMC article. Review.
References
-
- Tisdale MJ 1997 Biology of cachexia. J Natl Cancer Inst 89:1763–1773 - PubMed
-
- Larkin M 1998 Thwarting the dwindling progression of cachexia. Lancet 351:1336 - PubMed
-
- Plata-Salamán CR, Oomura Y, Kai Y 1988 Tumor necrosis factor and interleukin-1β: suppression of food intake by direct action in the central nervous system. Brain Res 448:106–114 - PubMed
-
- Plata-Salamán CR 1996 Anorexia induced by activators of the signal transducer gp 130. Neuroreport 7:841–844 - PubMed
-
- Sonti G, Ilyin SE, Plata-Salamán CR 1996 Anorexia induced by cytokine interactions at pathophysiological concentrations. Am J Physiol 270:R1394–R1402 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

