Integral membrane proteins Brr6 and Apq12 link assembly of the nuclear pore complex to lipid homeostasis in the endoplasmic reticulum
- PMID: 20016074
- PMCID: PMC2794714
- DOI: 10.1242/jcs.055046
Integral membrane proteins Brr6 and Apq12 link assembly of the nuclear pore complex to lipid homeostasis in the endoplasmic reticulum
Abstract
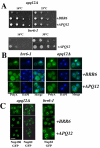
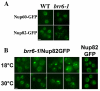
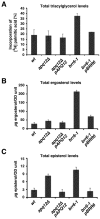
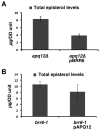
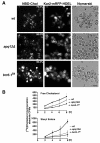
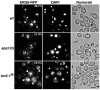
Cells of Saccharomyces cerevisiae lacking Apq12, a nuclear envelope (NE)-endoplasmic reticulum (ER) integral membrane protein, are defective in assembly of nuclear pore complexes (NPCs), possibly because of defects in regulating membrane fluidity. We identified BRR6, which encodes an essential integral membrane protein of the NE-ER, as a dosage suppressor of apq12 Delta. Cells carrying the temperature-sensitive brr6-1 allele have been shown to have defects in nucleoporin localization, mRNA metabolism and nuclear transport. Electron microscopy revealed that brr6-1 cells have gross NE abnormalities and proliferation of the ER. brr6-1 cells were hypersensitive to compounds that affect membrane biophysical properties and to inhibitors of lipid biosynthetic pathways, and displayed strong genetic interactions with genes encoding non-essential lipid biosynthetic enzymes. Strikingly, brr6-1 cells accumulated, in or near the NE, elevated levels of the two classes of neutral lipids, steryl esters and triacylglycerols, and over-accumulated sterols when they were provided exogenously. Although neutral lipid synthesis is dispensable in wild-type cells, viability of brr6-1 cells was fully dependent on neutral lipid production. These data indicate that Brr6 has an essential function in regulating lipid homeostasis in the NE-ER, thereby impacting NPC formation and nucleocytoplasmic transport.
Figures



References
-
- Aebi, M., Clark, M. W., Vijayraghavan, U. and Abelson, J. (1990). A yeast mutant, PRP20, altered in mRNA metabolism and maintenance of the nuclear structure, is defective in a gene homologous to the human gene RCC1 which is involved in the control of chromosome condensation. Mol. Gen. Genet. 224, 72-80. - PubMed
-
- Antonin, W., Ellenberg, J. and Dultz, E. (2008). Nuclear pore complex assembly through the cell cycle: regulation and membrane organization. FEBS Lett. 582, 2004-2016. - PubMed
-
- Cole, C. N., Heath, C. V., Hodge, C. A., Hammell, C. M. and Amberg, D. C. (2002). Analysis of RNA export. Methods Enzymol. 351, 568-587. - PubMed
-
- Conti, E., Muller, C. W. and Stewart, M. (2006). Karyopherin flexibility in nucleocytoplasmic transport. Curr. Opin. Struct. Biol. 16, 237-244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

